Exercise 6A: Determination of Soil Lime Requirements
Most plants grow best when the soil pH is near neutral. But, as noted in the preceding exercises, there is a tendency for the soils of humid regions to become acid as they get older and as they are used for growing crops. Excess acidity interferes with the availability of certain plant nutrients either by causing insoluble compounds to precipitate (such as iron and aluminum phosphates) or by altering decomposition processes (low pH slows the overall activity of microorganisms). Acid conditions also indicate that a considerable part of the soil’s cation exchange capacity is occupied by acidic cations rather than by plant nutrients such as calcium, magnesium, and potassium.
Lime is applied to the soil to counteract this acidity. Liming agents are compounds that consist of a cation (Ca2+ or Mg2+) to replace H+ (or H+ generated from other acidic cations) on the cation exchange sites and an anion (such as CO32-, 02-, or OH–) that will react with this H+ to form water. Some examples of liming agents are:
- CaCO3 — Calcium carbonate (calcitic limestone)
- MgCO3 — Magnesium carbonate (magnesite)
- CaO — Calcium oxide (burnt lime)
- Ca(OH)2 — Calcium hydroxide (slake or builder’s lime)
- CaMg(CO3)2 — Calcium-magnesium carbonate (dolomitic limestone)
The amount of lime required for neutralizing acidity usually is measured in tons per acre rather than in the smaller amounts most commonly needed for fertilizer applications. Liming involves large amounts because it is aimed at amending the chemistry of the soil rather than serving as a supplemental source of deficient plant nutrients. Lime is therefore classed as a soil amendment rather than a fertilizer, even though essential nutrients are added when lime is applied.
Fortunately, soil acidification is a gradual process. Soils that need lime once will undoubtedly need lime again, but it will probably take several years for the need to build up. Actually, the neutralization brought about by the lime is also a gradual process. It may take a year or two to bring the soil to the maximum pH it will attain before it begins to decline again. For this reason, the ideal time to apply lime (in a crop rotation) is about a year before the crop that will show the greatest responses to an elevated pH. Forage legumes such as alfalfa or sweet clover are likely to be the most responsive crops to liming in most rotations. The difference in lime requirements for row crops and forage legumes is sufficiently great that Iowa State University recommends liming to pH 6.9 if forage legumes are to be grown but only to pH 6.5 otherwise. Liming to pH 6.5 also is recommended if the time span considered is short (such as a 3-year lease). The economic benefits of liming to the higher pH are likely to require several years to repay the added costs (such benefits are usually detectable for 10 years or more).
In some locations, liquid lime is available as a manufactured or waste product. Because of size of particles, the reaction time usually is short, but there is little long-term residual neutralizing capacity. Also, one may pay a premium for this quick-reacting material because of the added weight of water to be handled.
The lime requirement of soils can be determined, or at least estimated, in several different ways. Two laboratory methods and one field method will be demonstrated in this exercise. One laboratory method uses information from the exercises on cation exchange capacity and/or those on exchangeable bases and exchangeable acidity; the other is a continuation of the exercise on soil pH and uses a pH meter to check the pH of a mixture of soil and a pH buffer. The pH of the buffer is changed by the soil; the amount of this change is used as a guide to the lime requirement. This second method is well adapted for rapid processing of large numbers of samples and is therefore commonly used in soil testing laboratories. This method measures both active and reserve acidity.
The field method is less exact than the laboratory methods of determining lime requirement but may be useful in developing countries where more precise methods are unavailable. Field evaluations of the clay and organic matter contents of the soil are used to estimate the cation exchange capacity. The lime requirement then can be approximated from the cation exchange capacity and the pH of the soil. Soil pH is often determined with colorimetric indicators when this method is used in the field.
No matter which method is used, the lime requirement is expressed in terms of effective calcium carbonate equivalent (ECCE). This unit of measurement is explained in the exercise on calcium carbonate equivalent.
Procedure
- Lime Requirement Estimates Based on pH, Texture, and Organic Matter (Field Estimate Method).
- Enter the soil pH on the answer sheet as determined by the indicator method and subtract that pH from 6.5 and 6.9 for the Δ pH values.
The indicator pH should be used because this is intended to be a field estimate.
- Estimate the clay content of the soil.
This estimate is made by moistening the soil to where it can be molded best and determining how long a 1/8-inch thick ribbon can be squeezed out between thumb and forefinger and still be self-supporting; alternatively, the soil may be rolled into wires 1/8 inch in diameter. The following may be used as a guide:
-
- 0 – 5% clay — soil does not hold a ball
- 5 – 10% clay — no significant ribbon, but soil will hold together
- 10 – 20% clay — 1 – 2 inch ribbon
- 20 % clay — approximately 2 inch ribbon that is weak
- 30% clay — >2 inch ribbon that holds a shape
- 40% clay — >2 inch ribbon stands straight up
- Multiply by the proper factor for the type of clay in the soil to convert percentage clay to meq/100g.
The following factors may be used (or in-between values for mixtures of clays). In Iowa, assume a smectite-illite mixture unless known differently.
| meq/100 g | meg/% | |
| Smectite | 80 | 0.80 |
| Smectite-illite mixture | 60 | 0.60 |
| Illite or chlorite | 30 | 0.30 |
| Kaolinite | 8 | 0.08 |
- Estimate the organic matter content of the soil from its color when the soil is moist.
The following may be used as a guide and improved upon with local experience (estimating by color has low accuracy as organic matter contents increase above 7%).
-
- 1% organic matter — yellowish brown
- 3% organic matter — dark brown
- 5% organic matter — brown-black
- 7% or more — black
- Multiply the percentage organic matter by 2.0 for an estimate of the cation exchange capacity contributed by the organic matter.
This factor assumes that the pure organic matter has a CEC of 200 meq/100 g, which is a reasonable estimate if in an oxidized form such as found in most soil humus (a raw peat saturated with water would have a much lower CEC).
- Add the cation exchange values for the clay and organic matter together to estimate the CEC of the soil. Then estimate the lime requirement by means of the equation:
Lime requirement (lb ECCE) = Δ pH x CEC x plow depth (inches) x 20
The equation is a field estimate using general Iowa conditions. The relationship is somewhat curvilinear and varies with the type of clay. The plow depth may be taken as 6 inches if the actual depth is not known.
NOTE: If your current pH is above 6.5 by indicator, subtract 1.0 pH unit from your average pH and use the equation above to calculate lime requirement.
- Lime Requirement Calculated from Base Saturation (Base Saturation Method).
- Calculate the present base saturation from the results of the exercises that deal with exchangeable base cations and exchangeable acidity (units are meq/100 g soil).
Cation exchange capacity (CEC) = exchangeable bases + exchangeable acidity
Present base saturation (PBS) = exchangeable bases / CEC × 100
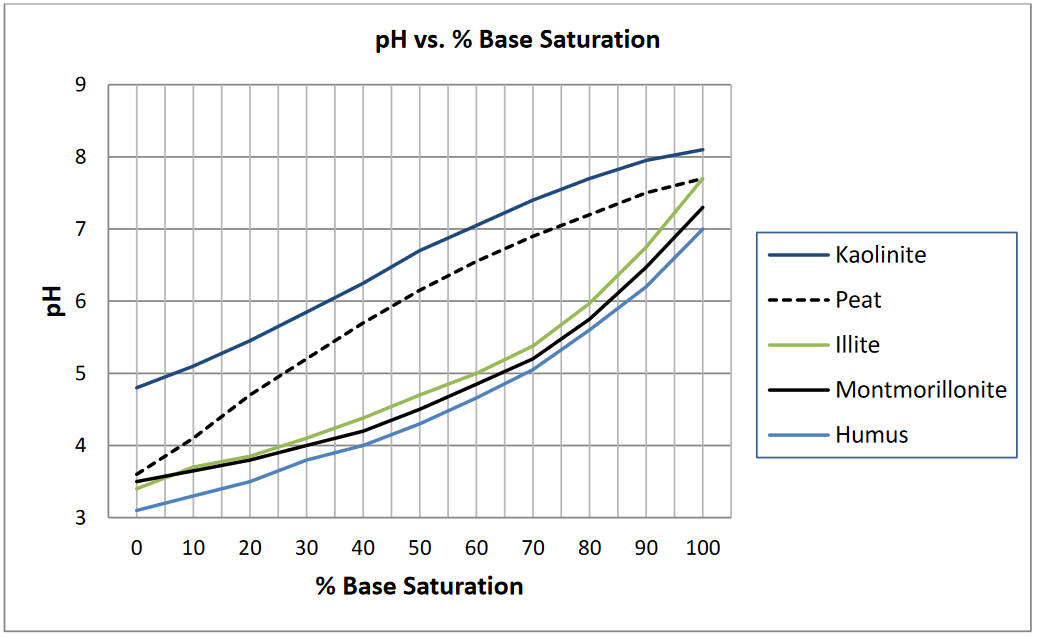
- Determine the desired base saturation (DBS).
Values can be read from the appropriate curve in Figure 1 if the dominant type of colloid is known or from a similar curve if one is available for local soils. Values of 95% base saturation for pH 6.9 or 90% base saturation for pH 6.5 can be used for many of the soils of North Central United States that have humus, montmorillonite, and illite clays as the main sources of CEC.
- Calculate the lime requirement per 2,000,000 lb of soil:
Lime requirement in ECCE (lb per acre) = (DBS – PBS)/100 × CEC × 1000
The factor 1000 is the product of the milliequivalent weight of calcium carbonate (50) multiplied by the factor for changing mg/100 g to pounds per 2,000,000 pounds (20). Adjustments should be made if the plow layer to be limed varies markedly from 2,000,000 lb/acre (6 inch depth).
NOTE: If your present percent base saturation is above 90%, subtract 10% from your present percent base saturation and use this value in the equation to calculate lime requirement.
- Lime Requirement by the Buffer Solution Method.
- Determine the soil pH with a pH meter (as described on page 25 of Exercise 5).
This procedure is described in the exercise on soil pH; the samples from the pH determination can be used here. A pH meter is needed both for accuracy and for use in the presence of the strongly colored buffer solution. Lime requirement is determined for any soil that has a pH sufficiently low that lime is required (pH 6.5 or below at most soil testing laboratories; below pH 6.6 for the purpose of this exercise).
NOTE: If your soil pH with a pH meter is 6.6 or higher, do not continue with this exercise. Instead, assume a buffer pH of 1.0 pH unit lower than your measured soil pH and use this value to determine lime requirement.
- Add 15 ± 0.1 mL of Sikora buffer solution to each beaker containing 15.0 ± 0.1 g of soil and 15 mL of deionized water. Stir thoroughly for 10 min.
This method measures both active and reserve (potential) acidity. The potassium ions replace the acidity ions on the cation exchange sites. The acidic ions are then in solution where they can be measured by the pH glass electrode. With the known buffering capacity of the Sikora solution, the depression of pH relates to lime requirement.
- Let stand for 10 min and determine the soil-buffer pH with the pH meter immediately after stirring using the procedure outlined for pH determination.
The lime requirement of the soil is indicated by the extent of pH drop below the original pH 7.7 of the buffer solution. The greater the total (active plus reserve) acidity of the soil, the greater the lowering of the buffer pH, and the greater the quantities of lime required to neutralize the acidity.
- Find the soil-buffer pH and the corresponding lime requirement for a 6-inch plow layer in Table 1. Enter the values on the answer sheet for the amount of ECCE needed to reach pH 6.5 and 6.9. If adding to a 7-inch depth, increase this amount proportionately or decrease proportionately if adding only to a 5-inch depth.
Each 0.1 unit of pH change in the buffer represents approximately 0.83 meq of exchangeable acidity per 100 g of soil. How does the acidity determined by the drop in buffer pH in this exercise compare with your earlier measured value for total acidity?
- Discard the soil-buffer solution in the container designated by the instructor.
| Soil-buffer pH | ECCE to raise soil pH to 6.5 lb/acre |
ECCE to raise soil pH to 6.9 lb/acre |
| 7.0 | — | 1100 |
| 6.9 | — | 1900 |
| 6.8 | 600 | 2700 |
| 6.7 | 1300 | 3500 |
| 6.6 | 2100 | 4400 |
| 6.5 | 2800 | 5200 |
| 6.4 | 3500 | 6000 |
| 6.3 | 4200 | 6800 |
| 6.2 | 5000 | 7700 |
| 6.1 | 7000 | 8500 |
| 6.0 | 6400 | 9300 |
| 5.9 | 7100 | 10,100 |
| 5.8 | 7900 | 11,000 |
| 5.7 | 8600 | 11,800 |
*If a tillage depth other than 6 inches is desired, the lime requirement is adjusted proportionately for depth. For example, if the lime for 6 inches is 2800 lb ECCE but it is to be worked into 8 inches of soil, more lime is needed. The amount needed can be determined by:
6/8 = 2800/X;
solving for X = 3733 lb ECCE
This says 6 is to 2800 lb as 8 is to 3733 lb.
EXERCISE 6A: DETERMINATION OF SOIL LIME REQUIREMENTS
Name____________________
Date_____________________
Section__________________
Soil number ________
| Soil Property | Desired soil pH | |||
| Actual | Estimated | 6.5 | 6.9 | |
| Field Estimate Method | ||||
| Soil pH by indicator solution | ||||
| pH change to reach 6.5 | ||||
| pH change to reach 6.9 | ||||
| Estimated clay content (%) | ||||
| Est. clay contribution to CEC (meq/100 g) | ||||
| Estimated O.M. content | ||||
| Est. O.M. contribution to CEC (meq/100 g) | ||||
| Estimated soil CEC (meq/100 g) | ||||
| Est. lime requirement (lb ECCE/acre) | ||||
| Base Saturation Method (calculate CEC values from Exercises 3 and 4) | ||||
| Cation exchange capacity (meq/100 g) | ||||
| Percent base saturation (%) | ||||
| Desired base saturation (%) | ||||
| Lime requirement (lb ECCE/acre) | ||||
| Sikora Buffer Solution Method | ||||
| Average soil pH by meter (from Ex. 5) | ||||
| Average soil-buffer pH (from Ex. 5) | ||||
| Lime requirement (lb ECCE/acre) 6 inches | ||||
| Lime requirement (lb ECCE/acre) 7 inches | ||||
Notes or comments (may continue on back or use separate page):

