Exercise 14: Determination of Available Potassium in Soils
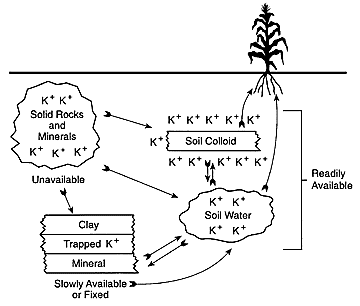
Soils normally contain a small amount of potassium in solution while a much larger amount of potassium is held on cation exchange sites, and a still larger amount in non-exchangeable forms, i.e. potassium held mostly between the layers of micas or in potassium feldspars. Available potassium includes the solution potassium, which is readily available for plant growth, and the exchangeable form, which is readily available by exchange reactions. Non-exchangeable potassium, on the other hand, is gradually converted into available forms but at a slow process. The rate of this conversion process is reasonably proportional to the amount of exchangeable potassium present in the soil. Annually, this process replenishes much of the potassium lost by crop removal and leaching (if any).
Both solution and exchangeable potassium can be removed from the soil by leaching with a salt solution that contains large amounts of a cation that can exchange with potassium. In an earlier exercise, we used Mehlich-3 to measure soil exchangeable bases that included potassium. Another common procedure, and the more traditional procedure, is to use 1 N ammonium acetate as the extracting solution, which we will use in this exercise. Both Mehlich-3 and 1 N ammonium acetate can be used to measure exchangeable cations from acids soils; however, Mehlich-3 is not recommended as a substitute for 1 N ammonium acetate as an extracting solution for Ca and Mg from calcareous soils.
The ammonium ion matches the charge and is nearly the same size as a potassium ion. The exchange process follows the principle of mass action. In this particular application, the principle states that an overwhelming concentration of ammonium ions in solution will replace practically all potassium ions on the cation exchange sites. It is therefore essential to have a large excess of ammonium ions present to prevent some potassium ions from returning to exchange sites.
Once the potassium ions are in solution, the concentration must be determined. Most simple potassium compounds are soluble and form colorless solutions. It is therefore difficult to determine the amount of potassium present in solution by direct chemical means. One available method of this type allows the formation of a fine precipitate of potassium cobaltinitrite, which is sometimes used in quick tests for plant tissue and soil samples. This method yields approximate results but is not fully quantitative.
Another method uses a flame photometer that makes it possible to measure the amount of potassium in solution without forming a precipitate. The potassium solution is fed into a flame at a controlled rate. The flame excites certain electrons in the potassium ions. When these electrons fall back into their usual orbits, they emit a characteristic red light at wavelengths of 766 and 769 nanometers. The flame photometer is equipped with a metering system to measure the intensity of light of this color (or other colors produced by other elements by proper adjustment).
A common method of measuring potassium in solution is by emission spectroscopy. Flame emission may be conducted with a flame photometry or with an atomic absorption spectrophotometer set on emission mode. In this procedure, a solution containing potassium atoms is passed into a flame. The flame excites the atoms and raises them to a higher energy state. When the potassium atoms cool and fall to their unexcited or ground energy state, they emit a specific wavelength of light. This specific wavelength of light can be isolated and its intensity measured. The greater the amount of potassium atoms in solution, the greater the emission of light. A standard calibration curve is then used to relate light emission to concentration. Emission is often the preferred method for measurement of potassium because it has a lower detection limit (more sensitive) than atomic absorption.
In atomic absorption spectroscopy, the amount of energy (in the form of light) absorbed by free potassium atoms in the gaseous state is measured. The electrons of the potassium atoms in flame are raised to higher orbitals (excited state) for a short period of time (nanoseconds) by absorbing a defined wavelength, which is produced by a hollow-cathode lamp. Potassium, as other elements, has its own distinct pattern of wavelengths at which it will absorb energy based on its unique configuration of electrons in its outer shell. As the concentration of potassium in the sample increases, absorption will also increase proportionally. Standard curves are then prepared to determine mg/L in solution of the samples. Atomic absorption spectroscopy can be used to determine over 60 different elements and is commonly used for calcium and magnesium determinations.
Like other chemical tests for soil fertility, the potassium test must be related to plant responses in the field. Experiments run for this purpose have revealed two effects that must be considered unique for potassium. One of these is the effect of aeration on plant uptake of potassium. Poorly aerated soil (usually caused by poor drainage) must contain larger amounts of exchangeable potassium than better aerated soils need for equivalent plant nutrition. This difference is managed by increasing the potassium fertilizer recommendation for poorly drained soils.
The other effect is that of air drying on the availability of potassium. Testing a soil before and after air drying will frequently show a difference in exchangeable potassium in the air-dried sample. Unfortunately, the amount of change that occurs with air drying is unpredictable. When the soil must be air dried, it should be done at room temperature and several days should be allowed for the soil potassium to reach a new equilibrium.
In some soils (mainly those with low to very low cation exchange capacity), the percentage of potassium saturation is also helpful in determining the amount of fertilizer potassium needed. In many other soils (especially those with medium to high cation exchange capacity), the use of percentage potassium saturation can lead to uneconomic use of fertilizer because no crop yield response is obtained for these additional amounts of fertilizer potassium.
Procedure
This is a common way in the past to determine extractable K but many Midwest laboratories today use Mehlich-3 extraction. We will do the calculations using the Mehlich values for K from Exercise 3.
- Weigh in duplicate 1.00 ± 0.05 g (record exact weight) of previously dried (95-105°C) soil in a 50-mL centrifuge tube. Add 10.0 ± 0.1 mL of neutral 1 N ammonium acetate solution and shake the flask for 5 min by hand.
Cation exchange processes are partly dependent on pH, so the solution should be neutral in reaction. A large excess of ammonium ions is provided to remove nearly all of the exchangeable potassium.
- 3.Determine the concentration of K in solution by using flame emission spectrophotometry and record. Extracts may need to be diluted as directed by instructor.
- 4.Calculate the mg/kg of K in soil.
Note that the factor used to convert ppm K in solution to ppm K in soil is 20/2, or 10.
- Determine the soil test category of your soil for K.
To make this classification, use the data for dried soil as presented in Table 9 found in Exercise 13 of this manual.
- Determine the lbs of K2O per acre required for corn grain production.
Use Table 10 found in Exercise 13 to determine the lbs of K2O required per acre for corn grain production. For this example, the soil is Clarion (fine textured) and information from Table 15 of PM 1688 (not shown) indicates that Clarion has Low Subsoil K.
EXERCISE 14: DETERMINATION OF AVAILABLE POTASSIUM IN SOILS
Name____________________
Date_____________________
Section__________________
Soil number________
| Replicate 1 | Replicate 2 | |
| Weight of soil (g) | ||
| Volume of extracting solution (mL) | ||
| Dilution factor | ||
| Emission of diluted solution | ||
| K in original or undiluted solution (mg/L) | ||
| K in soil (mg/kg) | ||
| Average K in soil (mg/kg) | ||
| Soil test class for K (from Table 9) | ||
| Lbs/ac of K2O needed for corn grain production (from Table 10) at low and high subsoil K | ||
Notes or comments:

