32 Acid Soils
Rivka Fidel
- Explain how soil acidification occurs naturally
- Describe how human activities contribute to soil acidification
- Explain how aluminum contributes to soil acidity and how it affects plant growth
- State the optimal pH range for plant growth and why
A Closer Look at pH
You may have learned elsewhere that acidic solutions and soils have a pH < 7, and basic or alkaline solutions and soils have a pH > 7. Now consider that pH is calculated as:
pH = -log[H+]
Thus, pH is the negative log of the hydrogen ion concentration. This means that as the H+ concentration increases, pH decreases. Hence an acid soil has more H+ ions than an alkaline soil. Additionally, the logarithmic scale of pH means each pH unit has 10x fewer H+ ions than the last. In other words, a solution with pH 8 has 1/10th the hydrogen ions than a solution with pH 7. And, a solution with pH 5 has 10x more hydrogen ions than a solution with pH 6.
Soil Reaction Class pH’s
Now, it’s not just whether pH is greater than or less than 7 that matters to plants and soil organisms. When soil pH fits into more specific ranges, we start to see specific effects on soil processes, as well as the growth of plants and microorganisms. For this reason, the USDA names specific soil pH ranges soil reaction classes. These reaction classes help us identify when plant growth limitations are likely.
|
USDA Soil Reaction Class
|
pH range | Growth of average plants* |
|---|---|---|
|
Ultra acidic
|
< 3.5 | Extremely limited |
| Extremely acidic | 3.5–4.4 | Very strongly limited |
|
Very strongly acidic
|
4.5–5.0 | Strongly limited |
| Strongly acidic | 5.1–5.5 | Moderately limited |
|
Moderately acidic
|
5.6–6.0 | Slightly limited |
| Slightly acidic | 6.1–6.5 | Not limited |
|
Neutral
|
6.6–7.3 | Not limited |
| Slightly alkaline | 7.4–7.8 | Slightly limited |
|
Moderately alkaline
|
7.9–8.4 | Moderately limited |
| Strongly alkaline | 8.5–9.0 | Strongly limited |
|
Very strongly alkaline
|
> 9.0 | Very strongly limited |
*Estimated level of limitation to the growth of average plants is used here for conceptual purposes only (by Rivka Fidel, University of Arizona).
As shown in the table, average plants grow best between pH 6.1–7.3. Other sources cite ranges of 6-7 or even 5.5–7.5, because the definition of an “average plant” varies. Many plants tolerate wide ranges of pH’s, whereas others are more selective. Apple trees for example grow well from pH 5–7.5, whereas pomegranates are more sensitive and need pH 6–6.9. Even more narrow ranges such as 6–6.5 are frequently considered optimal for some agronomic crops like corn and wheat (see here for New York examples and here for Iowa examples [p. 11]). Overall, plants tend to tolerate slightly acidic pH’s better than slightly alkaline pHs. This is due to the effect of pH on nutrient availability.
Problematic Acid Soils
Because most plants can slightly or even moderately acidic conditions well, here we will focus problematic acid soils – here defined as soils having a pH < 5.5 (i.e. strong acidity or greater). Below pH 5.5, plants are more likely to experience metal toxicity and/or nutrient deficiencies.
How Problematic Acid Soils Develop
Acid soils, including problematic acid soils, develop through a process called acidification. During acidification, acid cations (H+ and Al3+) enter the soil and lower the soil pH. Concurrently, H+ and Al3+ in solution adsorb to soil surfaces, replacing the nonacid cations there.
Acid cations (mainly H+) can come from outside the soil as inputs, or from chemical reactions occurring in soil. There are 5 main soil acidification processes, of which the first two are natural:
- Organism Metabolism
- Chemical weathering and decomposition
- Acid rain from fossil fuel combustion
- Excess CO2, also from fossil fuel combustion
- Excavation of S-rich sediments
- Fertilizer addition
Natural Causes of Acidification
Organism Metabolism
Organisms in soil produce both organic acids and carbon dioxide (CO2) as part of their metabolic processes, both of which lower soil pH. Plant roots can produce both H+ ions and organic acids to help them get nutrients from soil. The organic acids, primarily weak acids, donate H+ to the soil and thereby lower the pH. Microbes additionally produce organic acids as byproducts of their metabolism, and/or to help them break down organic matter and/or minerals.
Plants roots and soil microbes both produce CO2 as a byproduct of aerobic respiration. This CO2 then reacts with water to produce carbonic acid in a process called the carbonation reaction:
CO2(aq) + H2O(l) ⇌ H2CO3(aq)
Then the carbonic acid (H2CO3) dissociates, forming bicarbonate and H+:
H2CO3(aq) ⇌ HCO3–(aq)+ H+(aq)
Finally the H+ lowers the pH, acidifying the soil.
Chemical Weathering and Organic Matter Decomposition
Oxidation reactions occurring naturally in soil frequently have H+ in their products, and therefore lower soil pH over time. Specifically, most acidifying redox reactions feature O2 as the oxidizing agent, and accepts electrons from another element such as N, Fe, or S. For example, nitrification – a reaction where N gets oxidized and O2 gets reduced – produces 2 H+ ions per N oxidized:
NH4+(aq) + 2O2(g) ⇌ NO3–(aq) + H2O(l) + 2H+(aq)
Anthropogenic Causes of Acidification
Human activity lowers pH through various means, with most of the impacts occurring at the local to regional scale.
Acid Rain
Of all of the anthropogenic acidification processes, acid rain has likely acidified the most soil total. Acid rain is rainfall having a pH of ~4-5. This is much lower than normal rain, which has a pH of ~5.5. Acid rain forms due to emissions of SOx and NOx gases arising during fossil fuel burning. SOx gases are SO2 and SO3; NOx gases are NO and NO2. These gases react with O2 and H2O in the atmosphere, forming sulfuric acid (H2SO4) and nitric acid (HNO3), respectively.
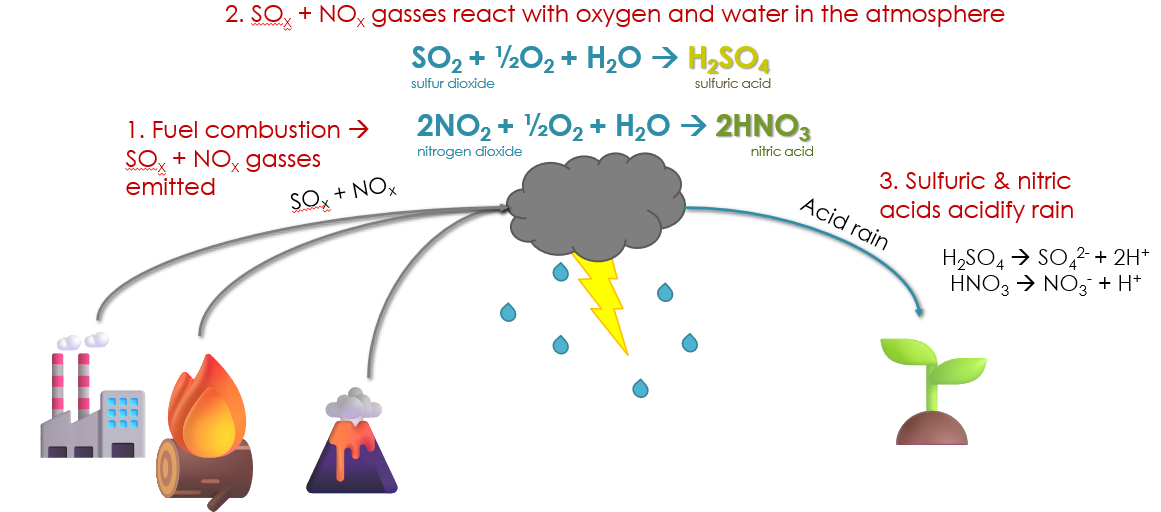
Among fossil fuels, coal – especially lignite coal – contains the most S, and hence produces the most SO2 emissions. For this reason, regions downwind of coal-fired power plants tend to experience the most acid rain. For example, the Midwestern US has historically had the most coal-fired power plants. Before the Clean Air Act of 1970 (especially amendments made in 1990), the SO2 from the power plants caused the downwind Eastern Midwest and Northeastern US to experience especially acidic acid rain. Since then, the rain has become less acidic and less extensive, but still occurs to this day.
Anthropogenic Carbon Dioxide Emissions
The burning of fossil fuels has raised CO2 concentrations beyond what aerobic respiration alone could achieve, causing greater acidification than could be accounted for naturally. Indeed, current CO2 concentrations of 410-420 ppm in 2025 are almost double the pre-industrial concentrations of ~280 ppm. This additional CO2 lowers the pH of water, and hence soil, in the same manner as CO2 from anywhere else: through the carbonation reaction (see above). However, because carbonic acid is a weak acid, it cannot lower the pH of rainfall below 5 like acid rain can. Hence it does not cause as much acidification as acid rain.
Excavation & Exposure of S-Rich Soils & Sediments
So-called “reduced forms” of sulfur – S0 , S22-, and S2- – are found in wetland soils and deep within the Earth. When wetland soils are drained for construction or agriculture, O2 gas enters the soil and elemental sulfur (S0) and sulfide (S2-) that naturally occur in the soil will readily react with the O2 to form sulfuric acid (H2SO4). When rocks and sediments bearing “reduced” sulfur forms are excavated during mining, the same reactions occur after they reach the surface and encounter O2 gas. Here are two example reactions:
S0(s) + 1.5O2(g) + H2O(l) ⇌ H2SO4–(aq)
S22-(aq) + 3.5O2(g) + H2O(l) ⇌ H2SO4–(aq) + SO42-(aq)
Because H2SO4 is a strong acid, it dissociates immediately and does not re-form:
H2SO4–(aq) ⇌ 2H+(aq) + SO42-(aq)
This raises the [H+] of the soil and lowers the pH.
Addition of Fertilizers
Some fertilizers are capable of donating electrons through oxidation reactions that yield H+, and thereby cause acidification over time. For example, ammonium (NH4+) is a popular nitrogen fertilizer that causes acidification through the aforementioned nitrification reaction. Adding S fertilizers containing S0 or S2- will likewise cause acidification when the S is oxidized and becomes sulfuric acid (H2SO4), per the above S oxidation reactions.
Where are Acid Soils Found?
Of the aforementioned acidifying processes, naturally occurring carbonation reactions and chemical weathering are by far the most widespread. Since these both occur faster in soils with sufficient moisture, and rain itself is acidic (even without human influence), we would expect soils in wetter environments to be more acidic. And indeed, the below soil pH and precipitation maps together show this is generally true.

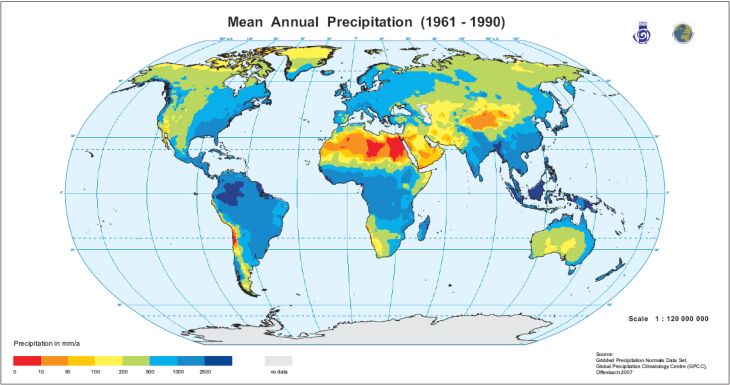
Moreover, when there is sufficient rainfall, more trees can grow, further acidifying the soil via the addition of H+ ions, CO2, and organic acids from their roots. As we learned in earlier chapters, conifers especially produce copious acids from their roots, and also have the most acidic leaves when compared with grasses and broadleaf trees. Hence, coniferous forests are the most acidic, followed by broadleaf forests, and then grasslands. Wetter forests such as rainforests also tend to be more acidic, as do peatlands. Local-scale impacts, as would be expected for peatlands and mine sites, are not easily discernable on global maps, but are nonetheless important.
Limits to Plant Growth in Acid Soils
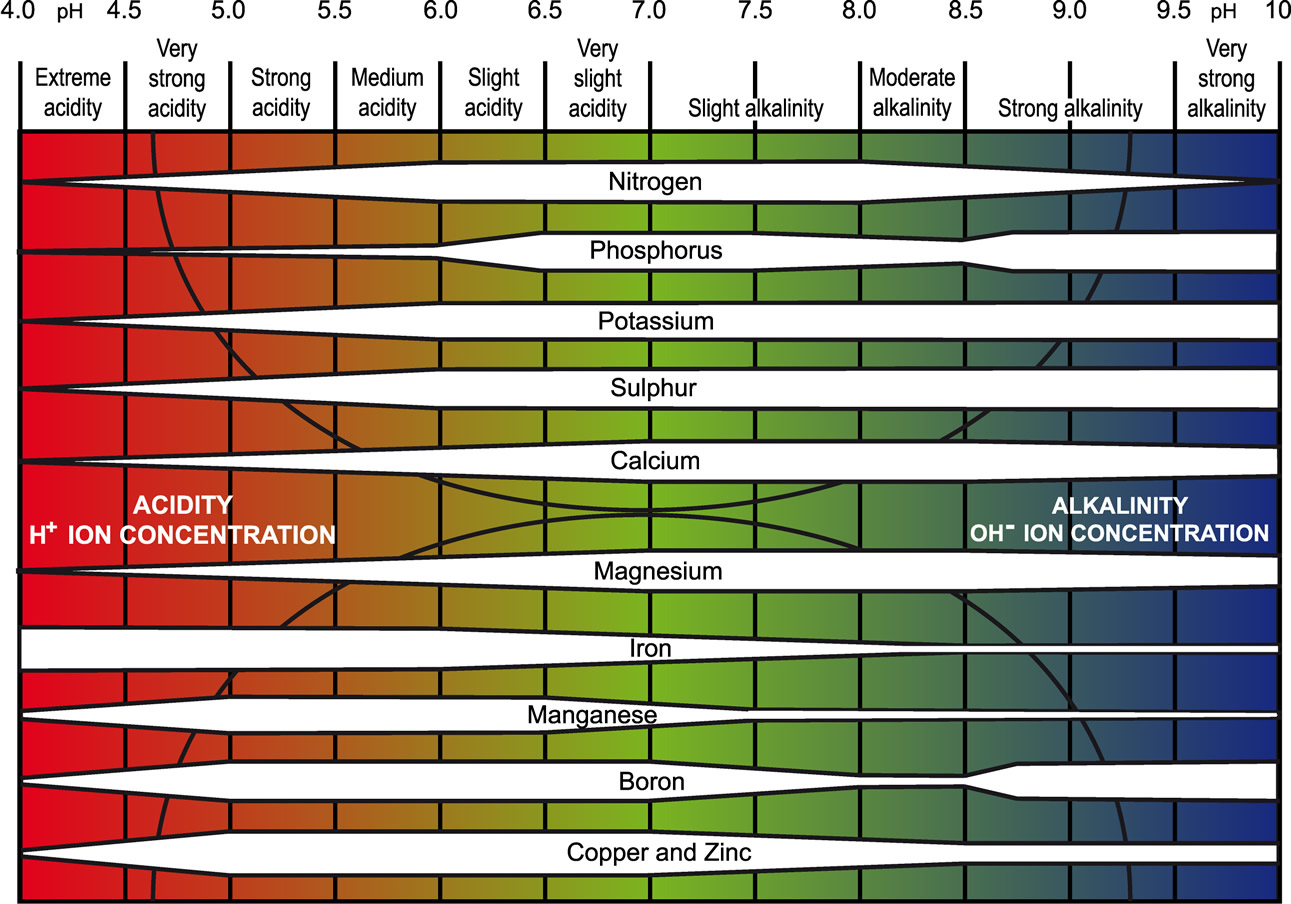
Soil pH primarily affects plant growth through its effect on nutrient availability. Only nutrients that located in the soil solution or adsorbed to exchange sites are available to plants. So, anything that increases nutrients’ solubility or the soil’s exchange capacity will also influence availability.
Metal Toxicities
Generally, transition metal micronutrients such as Fe, Mn, Cu, and Zn are more soluble at low pH’s, and may reach toxic concentrations. This is because they normally bond to alkaline anions like OH– and O2-, which have lower concentrations at low pH’s. When pH decreases, the oxide and hydroxide anions react with H+ to form water, making metal oxides and hydroxides more soluble. When pH falls below 5.5, transition metal micronutrients can reach toxic concentrations, essentially causing plants to “overdose” on these micronutrients.
Problematic acid soils (pH<5.5), also often contain toxic concentrations of Al3+. Al is a post-transition metal, and like transition metals, Al3+ is more soluble at lower pHs. However, Al is not a plant nutrient, but instead is toxic to plants. Thus when pH falls below 5.5, plants commonly experience aluminum toxicity.
More about Aluminum and Iron in Acid Soils
In addition to H+, aluminum III (Al3+) and iron (Fe2+ and Fe3+) are also acid cations because they undergo a type of reaction called hydrolysis. In hydrolysis, a metal accepts OH– from H2O, leaving behind H+ (see the acid and base saturation chapter for more information). For example, this is the aluminum hydrolysis reaction:
Al3+(aq) + 3H2O(l) ⇌ Al(OH)3(s) + 3H+(aq)
In problematic acid soils (pH<5.5), the abundance of H+ (and lack of OH–) helps reverse the aluminum hydrolysis reaction, favoring Al3+ dissolution, and causing Al3+ concentrations in solution to increase:
Al(OH)3(s) + 3H+(aq) ⇌ Al3+(aq) + 3H2O(l)
Likewise, the iron II & III hydrolysis reactions also reverse easily when pH < 5.5, causing Fe ion concentrations in solution to increase:
Fe(OH)2(s) + 2H+(aq) ⇌ Fe2+(aq) + 2H2O(l)
Fe(OH)3(s) + 3H+(aq) ⇌ Fe3+(aq) + 3H2O(l)
Fe2+ and Fe3+ are far less abundant in the solution compared with Al3+, so for practical purposes, scientists and growers normally focus on H+ and Al3+.
Nutrient Deficiencies and Low CEC
Recall that CEC declines with declining pH, lowering the capacity of soil to adsorb and retain nutrients. This means that exchangeable nutrient cations like Ca2+, Mg2+, and K+ are not retained well over time in acid soils, and instead leach out. Therefore, acid soils (especially when pH < 5.5) easily become deficient in these nutrients.
Additionally, the abundant Al3+, Fe2+ and Fe3+ found in solution when pH < 5.5 easily bond to and precipitate with phosphates (H2PO4–, HPO42–, PO43-) and reducing plant-available P. Here are some example reactions:
- Aluminum hydroxy phosphate precipitation: Al3+(aq) + H2PO4–(aq) + 2H2O(l) ⇌ 2H+(aq) + Al(OH)2H2PO4(s)
- Variscite precipitation: Al3+(aq) + PO43-(aq) + 2H2O(l) ⇌ AlPO4·2H2O(s)
- Strengite precipitation: Fe3+(aq) + PO43-(aq) + 2H2O(l) ⇌ FePO4·2H2O(s)
The solids in the products of these reactions are only poorly to slightly soluble. As a result, phosphorous availability decreases below pH 6.
At especially low pH’s, normally below 5, sulfur may also become deficient. This is because when sufficient positive charge develops on colloid surfaces, SO42- can start adsorbing to positive charge sites through inner-sphere adsorption mechanisms. Meaning, SO42- becomes very strongly bound to the surfaces, making it non-exchangeable, and no longer plant-available.
This table shows at about what pH deficiencies are likely to occur, and other conditions under which deficiencies become more likely (Data compiled by Jim Walworth, University of Arizona):
| Nutrient problem | Soil pH | Other soil conditions |
|---|---|---|
| Al and Mn toxicity | < 5.0 to 5.5 | depends on plant species and variety |
| Ca deficiency | < 4.5 to 4.8 | low CEC, tropical highly weathered soils |
| Mg deficiency | < 5.5 | low CEC or BS |
| Mo deficiency | < 5.5 | low solubility |
| N deficiency | < 5.0 to 5.5 | decreased nitrification & mineralization, low OM soils |
| P deficiency | < 5.0 | highly weathered soils, dominant in Al/Fe oxides |
| K deficiency | < 5.0 | low CEC, low BS, highly leached soils, high exchangeable Al |
- CEC = Cation Exchange Capacity
- BS = Base Saturation (see cation saturation percentages chapter)
- OM = Organic Matter
Summary of Problematic Acid Soil Plant Impacts
To summarize, problematic acid soils (pH < 5.5) limit plant growth as follows:
- Aluminum toxicity due to enhanced solubility of Al3+
- Excess available transition metal micronutrients (often reaching toxic levels): Fe2+, Mn2+, Cu2+ and Zn2+
- Low cationic macronutrients: Ca2+, Mg2+, and K+
- Low plant-available P due to PO43- precipitating with Al and Fe
Note the following about acid soils and acidification
- Distinguishing property: Low pH (excessive H+)
- Acid soil: pH < 7
- Problematic acid soil: pH < 5.5
- Formation conditions
- Acid soils are found in moist climates on the global scale, because rain accelerates acidification
- Acidification is the accumulation of H+ in the soil. There are several processes that acidify soil:
- Organism Metabolism
- Chemical weathering and decomposition
- Acid rain from fossil fuel combustion
- Excess CO2, also from fossil fuel combustion
- Excavation of S-rich sediments
- Fertilizer addition
-
Problematic acid soils (pH < 5.5) limit plant growth as follows:
- Low cation macronutrients: Ca2+, Mg2+, and K+
- High transition metal micronutrients (often reaching toxic levels): Fe2+, Mn2+ and Cu2+
- Low P due to PO43- precipitating with Al and Fe
- Aluminum toxicity due to enhanced solubility of Al3+

