33 Alkaline Soils
Rivka Fidel
- Identify common alkalis from a list of compounds found in soil
- Explain how and where alkaline soils form
- Describe how calcite-carbonate/rich soils are identified
- Explain which nutrients are frequently deficient in alkaline soils and why
- Explain why isn’t it economical to remediate alkaline soils in the field
- Describe how small quantities of alkaline soil (ex: in pots and raised beds) can be remediated
- List appropriate soil acidifiers for lowering soil pH
What is an alkaline soil?
In a chemical sense, alkaline soils simply have pH > 7, just like ordinary basic solutions. Recall that pH = -log[H+], so alkaline soils have low [H+], and consequently more OH- than H+. The more OH- (and less H+) a soil has, the greater its alkalinity.
What are alkalis?
Alkalis are basic salts that include an alkali or alkaline earth metal. Recall that bases accept H+ and thereby raise the pH. Salts are all ionic solids, composed of a metal cation and nonmetal anion. Therefore, we can also define alkalis as containing an alkali or alkaline earth metal, plus an anion that acts as a base. Here are some examples of alkalis and the ions that form when they dissolve:
| Alkali | Metal cation(s) | Nonmetal anion(s) (base(s)) |
|---|---|---|
| NaOH | Na+ | OH- |
| Ca5(PO4)3(OH) | Ca2+ | PO43- and OH- |
| CaCO3 | Ca2+ | CO32- |
| Ca,Mg(CO3)2 | Ca2+ and Mg2+ | CO32- |
Note that the metals in alkalis are all nonacid cations. Unlike Al3+ and transition metals, these are not capable of accepting OH- (this is why NaOH completely dissociates in water and does not re-form). The most common nonacid cation in soil is Ca2+, followed by Mg2+, K+, and Na+. The most common alkaline (basic) anion in soil, by far, is CO32-. Consequently, the most common alkali in soil is calcite (CaCO3).
How do alkaline soils develop?
Alkaline soils develop primarily in arid to semi-arid regions, as well as poorly drained areas, due to the lack of H+ additions from rain and/or also lack of alkali losses through leaching. When there is insufficient water flow to wash away alkalis, and insufficient acid to neutralize them, they accumulate in soil. Over time, these alkalis – primarily calcite – reach a sufficiently high concentration to increase the pH above 7.4, where we start seeing negative effects on average plants.
When calcite specifically accumulates, the resulting soils are called calcareous. Calcareous soils are very common in deserts wherever soil has had sufficient time to develop, and can also be found in areas experiencing dry winters and/or summers such as grasslands and Mediterranean shrublands. In the case of moist grasslands like in the Midwest, calcareous soils are commonly found low in the landscape, where the soil is poorly drained and receives dissolved or eroded carbonate minerals from upslope. During dry periods, the carbonate-rich water evaporates, leaving behind calcite and smaller amounts of other carbonate salts.
Calcareous soils also commonly develop from limestone or other carbonate-rich parent materials. In these soils, the topsoil grows more acidic over time, but the subsoil remains alkaline and calcareous. Then, when erosion carries away the more acidic topsoil, it may expose calcite-rich layers of soil or sediment at the surface.
Many calcareous soils have calcic horizons where calcite has accumulated over time in the subsoil (endopedon). Calcareous soils and calcic horizons can be identified using effervescence testing. Over time calcic horizons can become cemented as the calcite fills in the pores between particles, turning them into petrocalcic horizons.
- This soil in NE Kansas shows calcium carbonate accumulation lower in the profile, shown by the white masses. [Photo Credit: Amber Anderson]
- This soil near North Platte, NE, shows a calcium carbonate ‘rind’ on the soil due to a lack of leaching in loess materials. [Photo Credit: Amber Anderson]
- This petrocalcic, cemented horizon due to calcium carbonate is present in this Southern California aridisol from 35-80 cm. [Photo Credit: Amber Anderson]
- This soil in Iowa shows a clear development line approximately 2/3 down the profile. Material below this(lighter colored) is calcareous due to the parent material having free calcium carbonate, and a lack of development as the soil is too young. [Photo Credit: Amber Anderson]
Identifying Calcareous Soils: Effervescence Test
Calcite-rich soils and soils rich in other carbonates such as Na2CO3 can be identified using effervescence testing. To perform the test, simply add a few drops of 1 M HCl and watch for bubbles. More bubbles indicate more carbonates.
This table shows the different effervescence classes and their criteria (NRCS):
| Effervescence Class Terms | Abbreviation | Effervescence class Criteria |
|---|---|---|
| Non-effervescent | NE | No bubbles form |
| Very slightly effervescent | VS | Few bubbles form |
| Slightly effervescent | SL | Numerous bubbles form |
| Strongly effervescent | SE | Bubbles form a low foam |
| Violently effervescent | VE | Bubbles quickly form a thick foam |
The following reaction is responsible for the bubbles (the ↑ indicates gas escaping and bubbling):
CaCO3 + 2HCl → CaCl2 + H2O + CO2↑
Calcareous soils will bubble upon adding other acids as well, such as acetic acid, which is found in vinegar. However, vinegar isn’t strong enough to react with some of the less calcareous soils – in other words, it cannot detect low concentrations of carbonate salts. You can use it for at-home testing – handy when children/pets are present – but interpret results cautiously. Fewer bubbles are expected when using vinegar compared with HCl.
Similar to soil scientists, geologists use HCl to identify calcite and limestone specimens, but the test is normally used non-quantitatively in geology to simply indicate the presence or absence of carbonates.
Normally calcareous soils have a pH between ~7.5-8.5, no matter how much calcite has accumulated. This is because calcite is poorly soluble in neutral to alkaline water. Therefore, the low solubility of calcite limits the concentration of CO32- in the soil solution. However, in the salt-affected soils chapter, we’ll learn that the presence of excess sodium in alkaline soils increases the solubility of CO32-, allowing the pH to rise above 8.5.
When and why is soil alkalinity problematic for plants?
As with acidity, some plants are more sensitive to alkalinity than others, so it is hard to generalize impacts of alkalinity on all plants. From the reaction classes table in the acid soils chapter, you may have noticed that slightly alkaline soils (pH 7.4-7.8) have slight limitations to plant growth, moderately alkaline soils (pH 7.9-8.4) have moderate limitations to plant growth, and so forth. Soil pH can only exceed 8.5 when excess sodium is present; see information on sodic soils in the salt-affected soils chapter.
| Alkaline soil-intolerant crop examples | Slightly alkaline soil-tolerant crop examples | Alkaline soil-tolerant crop examples |
|---|---|---|
|
|
|
If we examine the plant nutrient availability diagram, we can see that the plant-availability of transition metal micronutrients starts declining above pH 7. The rate of decline is fastest in manganese (Mn), followed by iron (Fe), then copper (Cu) and zinc (Zn). So, we would expect that as pH increases, Mn deficiencies would occur first, then Fe deficiencies, and then Cu and Zn.
Most nutrient availability diagrams are generalized to all soil types, but most of the more alkaline soils are found in dry regions for the aforementioned reasons, and such soils are generally low in organic matter. Therefore, we’ve included an alternative set of nutrient availability diagrams below (different from the one appearing in the acid soils chapter), which show the difference between mineral vs organic soils. Outside of wetland soils, the mineral soils diagram on the left is most appropriate. The reason organic matter affects nutrient availability is because nutrients adsorb to organic matter at different pH’s compared to minerals due to its different surface chemistry. Likewise, among mineral soils, the minerology affects both the rate of nutrient sorption and precipitation of nutrients with other ions. Generally such diagrams should be interpreted cautiously since the actual effect of pH on soil nutrient availability depends on the minerology of the soil as well as the organic matter content and composition.
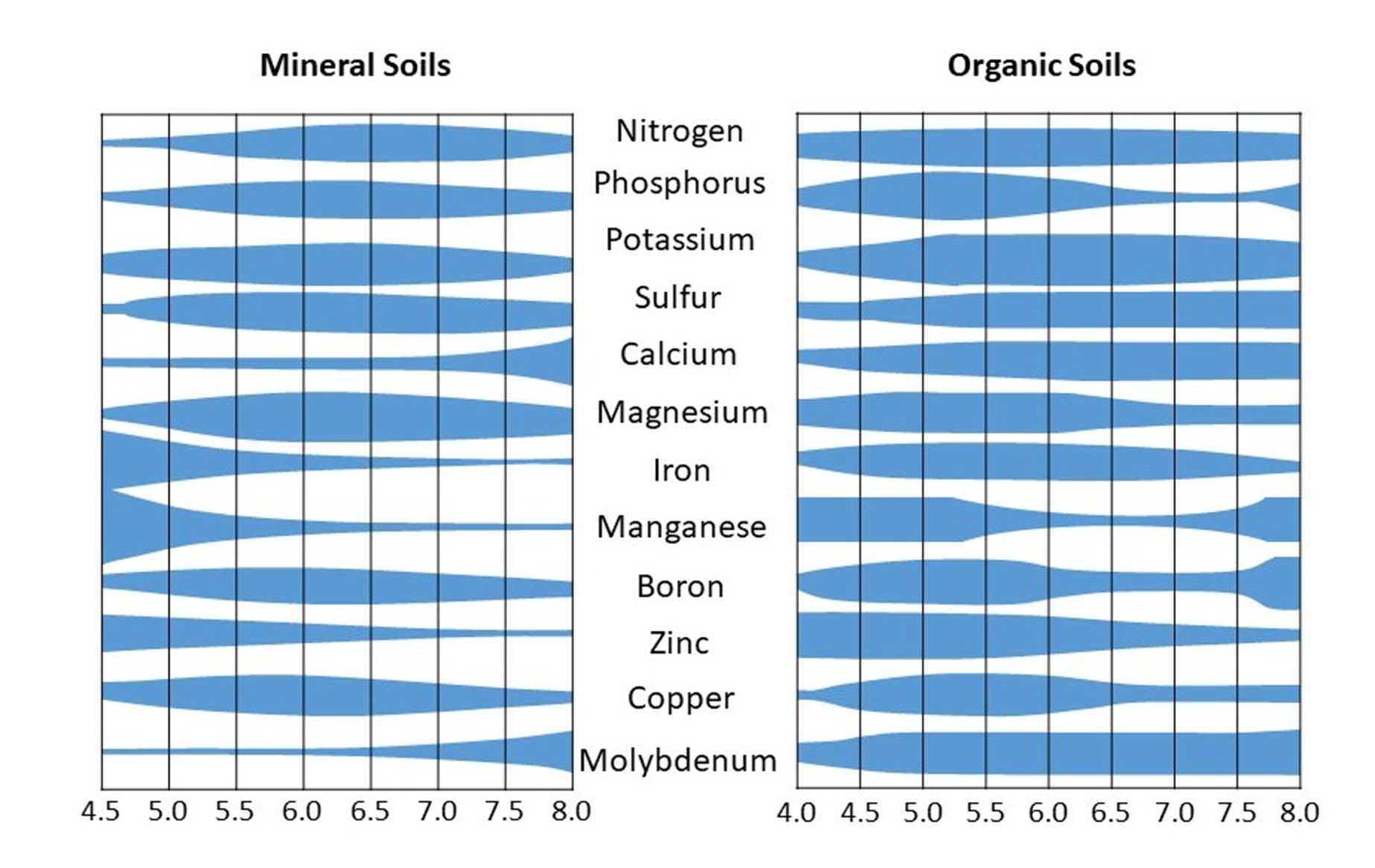
To summarize, alkaline soils present the following challenges to plant growth:
- Low transition metal micronutrient availability: Fe2+, Mn2+, Cu2+, and Zn2+
- Largely due to hydrolysis, i.e. precipitation with OH-
- Low P availability
- Largely due to PO43- precipitation with Ca2+ and Mg2+
Can we remediate alkaline soils?
Not in the field!
It is not normally economical to acidify alkaline soils in situ, i.e. the field. This is because alkaline soils (especially calcareous ones) tend to contain enormous amounts of calcite, and each mole of calcite (CaCO3) requires 2 moles of H+ to neutralize it. Furthermore, even if you acidify the topsoil, the subsoil will likely have even more calcite in it that may eventually move upwards as the groundwater table rises and falls.
Estimating Acid Requirement for an Alkaline Soil in Situ (in the field)
Consider 1 ha of soil with just 1% CaCO3 in the top 30 cm of soil. How much CaCO3 would it contain in total and how much H2SO4 would be needed to neutralize it? Assuming an average bulk density of 1.3 g/cm3, we need to first calculate how much CaCO3 it would have (1 Mg = 1 metric ton = 103 kg = 106 g) using unit conversions (highlights show cancelling units):
[latex]30 cm * \frac{10^{8} cm^{2}}{1 ha}*\frac{1.3 g\:soil}{cm^{3}\:soil} * \frac{1 Mg\:soil}{10^{6} g\:soil} * \frac{1 Mg\:CaCO_3}{100 Mg\:soil}[/latex]
=39 Mg CaCO3/ha
…and then use mole-mass ratios to determine how much sulfuric acid would be required to neutralize it (each mole of H2SO4 has 2 H+ so we don’t need to multiply by 2):
[latex]\frac{39Mg\:CaCO_3}{ha}*\frac{1\:mol}{100g\:CaCO_3}*\frac{98g\:H_2SO_4}{1mol}[/latex]
=38 Mg H2SO4/ha
These calculations don’t account for leaching losses, or how diluted H2SO4 has to be before it can be safely applied to soil. So, you’d have to apply even more H2SO4 solution compared with what we just calculated here. At the time of writing (2025), 0.725 Mg of technical grade sulfuric acid costs ~$1,000 (or more). Thus, even without accounting for leaching losses or dilution, you’d have to spend over $50,000 per hectare!
What if you want to add elemental S instead? Then less is needed since S weighs less, and 1 mol of S produces 1 mol of H2SO4 after reacting with O2 (see reactions in the below section).
[latex]\frac{39Mg\:CaCO_3}{ha}*\frac{1\:mol}{100g\:CaCO_3}*\frac{32g\S}{1mol}[/latex]
= 12.5 Mg S /ha
At the time of writing, you can buy 2000 lbs (0.907 Mg) of elemental S for $845. So, 12.5 Mg ÷ 0.907 Mg × $845 = $11,645. Even if you were to find a bulk discount, the costs would easily exceed $10k per hectare.
As you can see, the costs of acidifying soil in situ add up quickly, no matter what acidifying agent you use. Therefore it is normally not feasible to acidify alkaline soil to pH’s suitable for average crops (i.e. pH 7 or less) in the field.
…But we can acidify pots and raised beds
As a result of calcite chemistry and the enormous amounts of it in alkaline soils, it is only economical to acidify very small amounts of soil such as pots of soil or raised beds filled with soil. To lower the pH of small amounts of alkaline soil, the following can be added:
- Sulfuric acid: H2SO4
- Elemental sulfur: S0
- Acidic metal compounds: Al2(SO4)3 and FeSO4
Elemental sulfur and the acidic metal compounds do not themselves contain H+; instead a preliminary reaction in soil must occur first before the pH decreases. As a result, these latter two will lower the soil pH more slowly. The acid-generating reactions for these amendments are shown in the below table (the Al2(SO4)3 and FeSO4 are assumed to dissolve rapidly, so that the vast majority of the acid cations, Al3+ and Fe2+, end up dissolving).
| Soil acidifier | Acid-generating reaction in soil |
|---|---|
| S0 | S0(s) + 1.5O2(g) + H2O(l) ⇌ H2SO4(aq) |
| Al2(SO4)3 | Al3+(aq) + 3H2O(l) ⇌Al(OH)3(s) + 3H+(aq) |
| FeSO4 | Fe2+(aq) + 2H2O(l) ⇌ Fe(OH)2(s) + 2H+(aq) |
Elemental sulfur in particular requires a biologically-mediated redox reaction to acidify soil (the first reaction in the above table), and is known to frequently take a long time to lower the pH, especially in dry conditions. Its acidification rate is furthermore variable, depending on the weather and abundance of S-oxidizing microorganisms. Consequently, when at first the pH doesn’t go down sufficiently after S0 addition, it is best to wait longer for results, so as to avoid overshooting and accidentally over-acidifying the soil. Nonetheless, elemental S is relatively inexpensive and safe to use, with a minimal risk of adding excess Al or Fe, making it the most popular soil acidifier.
Calculating elemental S to add to alkaline soil in a raised bed
Let’s say you need to acidify an alkaline soil in a raised bed that is 1m3 in volume, and the soil has a bulk density of 1.2 g/cm3. If the % carbonate or calcite is unknown, we can use effervescence to get a very approximate estimate following Maschmedt 2004.
| Effervescence Class Terms | Abbreviation | Effervescence class Criteria | Approximate % carbonate |
|---|---|---|---|
| Non-effervescent | NE | No bubbles form | <0.5 |
| Very slightly effervescent | VS | Few bubbles form | 0.5-1.5 |
| Slightly effervescent | SL | Numerous bubbles form | 1.5-8 |
| Strongly effervescent | SE | Bubbles form a low foam | 1.5-8 |
| Violently effervescent | VE | Bubbles quickly form a thick foam | >8 |
Let’s say the soil was very slightly effervescent (VS), so for simplicity we’ll assume ~1% calcite from the table above. As with soil in the field, we need to calculate the mass of calcite (the term 1 g CaCO3/100g soil is equivalent to 1% calcite):
=12 kg/bed
Now we use mass-mole ratios to calculate the elemental S to add:
=3.8 kg S/bed
Prices for gardening-sized bags of elemental S vary but if we assume ~$1-2/lb ($0.5-1/kg) based on prices of 50 lb bags in 2025, then this would cost $2-4.
So we see that, in contrast to acidifying many acres or hectares of soil in the field, lowering the pH of alkaline soil in raised beds or pots can be relatively affordable.
- Alkaline soils have pH > 7 because they have more OH- than H+
- Alkaline soils gradually become more problematic to plant growth as pH rises; the degree of effect depends on the plant in question
- The most common alkali in soils is CaCO3, calcite
- Effervescence testing (adding acid and watching for bubbles) is used to test for calcite and other carbonates
- Alkaline soils present the following challenges to plant growth:
- Low transition metal micronutrient availability: Fe2+, Mn2+, Cu2+, and Zn2+
- Largely due to hydrolysis, i.e. precipitation with OH-
- Low P availability
- Largely due to PO43- precipitation with Ca2+ and Mg2+
- Low transition metal micronutrient availability: Fe2+, Mn2+, Cu2+, and Zn2+
- It is not usually economical to lower the pH of alkaline soils in the field
- The following soil acidifiers can be used to lower pH of alkaline soil in pots, raised beds, and other containers: H2SO4, S0, Al2(SO4)3, and FeSO4





