30 Cation Exchange Capacity (CEC) Calculations
Rivka Fidel
- Identify which CEC formula is appropriate to use with provided data
- Calculate cation exchange capacity using 3 approaches:
- Sum of exchangeable cations
- Weighted average of colloid CEC's
- Quantification of displaced cations
Ways to Measure CEC
CEC stands for cation exchange capacity, the total amount of cations a soil can retain, measured in cmolc/kg (or meq/100g, but here we’ll stick with cmolc/kg).
There are 3 ways to measure and calculate CEC:
- Quantify and sum up the exchangeable cations, in cmolc/kg
- Take a weighted average of important colloids’ known CEC’s
- Cation displacement:
- Replace all of the cations on the surface with NH4+
- displace the NH4+ with K+
- Measure the displaced NH4+ in solution
- Convert units NH4+ was measured in to cmolc/kg
Some instructors may require you to learn just one of these, whereas others may require all 3. Requirements for a lab course may differ from a lecture course.
Approach 1: Quantify and Sum Up Exchangeable Cations
Method
In the first approach, all exchangeable cations are extracted from the soil and then measured. There are various ways to do this, but here’s an example:
- Shake up soil with ~1 M BaCl2, SrCl2, or NH4Cl, or (NH4)2SO4 solution for 24h
- Allow to settle and filter the supernatant
- Analyze the filtered supernatant for common exchangeable cations in soil, namely: Al3+, Ca2+, Mg2+, K+, Na+ . This is often done with an atomic adsorption analyzer, ion selective electrode, or Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
- NH4+ can be analyzed only if it is not used in step 1
- H+ can be analyzed by measuring pH, but this “exchangeable” cation isn’t always exchangeable, and the method isn’t always accurate. The issues with the method go beyond the scope of this book, however.
For introductory courses at most institutions, you’ll simply be given these data, whereas analysis would wait until an upper-level course. So the important skill for now is learning how to calculate CEC from these data.
Calculation
When doing this calculation, pay close attention to the units. This will affect whether you need to do a conversion or not.
Units in Centimoles of Charge per Kilogram (cmolc/kg)
When units are in cmolc/kg, you just need to add up all of the cations. Let’s say only 4 cations are measured at the concentrations shown in this table:
| Cation | Concentration (cmolc/kg) |
|---|---|
|
Na+ |
2 |
|
K+ |
3 |
|
Mg2+ |
5 |
|
Ca2+ |
15 |
Since the concentrations are already in the desired units, we just have to add them up:
CEC = [Ca2+] + [Mg2+] + [K+] + [Na+] + [Al3+] + [H+]
= 2 cmolc/kg + 3 cmolc/kg + 5 cmolc/kg + 15 cmolc/kg + 0 cmolc/kg + 0 cmolc/kg
= 20 cmolc/kg
Note how we weren’t given concentrations of aluminum or hydrogen, so these were assumed to be zero.
Units in milligrams of cations per kg of soil (mg/kg)
Because different instruments and soil testing services report different units, you frequently will see other units besides cmolc/kg. The most common of these is mg/kg.
To convert from mg/kg to cmolc/kg, we need to use the molar mass from the periodic table, metric orders of magnitude, and the charge of each ion. Here are the steps in words:
- Divide by the molar mass
- Divide by 10 mmol per cmol (because there are 10 millimoles in a centimole)
- Multiply by the charge
Soil test results show that a soil contains the following cations in mg/kg: Ca2+ = 1850, Mg2+ = 800, K+ = 530, Na+ = 250, Al3+ = 120, and H+ = 20.
From the periodic table we find the following atomic masses in mg/mmol or g/mol (1 g/mol = 1 mg/mmol):
| Element | atomic mass (mg/mmol) |
|---|---|
| Ca | 40 |
| Mg | 24 |
| K | 39 |
| Na | 23 |
| Al | 27 |
| H | 1 |
Now we convert the units for each cation to cmolc/kg like this:
[latex]\frac{1850 \mathrm{mg} \mathrm{Ca}^{2+}}{\mathrm{kg} \mathrm{soil}} \times \frac{\mathrm{mmol}}{40 \mathrm{mg}} \times \frac{\mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{2 \mathrm{cmol}_c}{\mathrm{cmol}}=9.25 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
[latex]\frac{800\mathrm{mg} \mathrm{Mg}^{2+}}{\mathrm{kg} \mathrm{soil}} \times \frac{\mathrm{mmol}}{24 \mathrm{mg}} \times \frac{\mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{2 \mathrm{cmol}_c}{\mathrm{cmol}}=6.97 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
[latex]\frac{530\mathrm{mg} \mathrm{K}^{+}}{\mathrm{kg} \mathrm{soil}} \times \frac{\mathrm{mmol}}{39\mathrm{mg}} \times \frac{\mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{1 \mathrm{cmol}_c}{\mathrm{cmol}}=1.36\frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
[latex]\frac{250\mathrm{mg} \mathrm{Na}^{+}}{\mathrm{kg} \mathrm{soil}} \times \frac{\mathrm{mmol}}{23 \mathrm{mg}} \times \frac{\mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{1 \mathrm{cmol}_c}{\mathrm{cmol}}=1.09 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
[latex]\frac{120\mathrm{mg} \mathrm{Al}^{3+}}{\mathrm{kg} \mathrm{soil}} \times \frac{\mathrm{mmol}}{27\mathrm{mg}} \times \frac{\mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{3 \mathrm{cmol}_c}{\mathrm{cmol}}=1.09 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
[latex]\frac{20\mathrm{mg} \mathrm{H}^{+}}{\mathrm{kg} \mathrm{soil}} \times \frac{\mathrm{mmol}}{1 \mathrm{mg}} \times \frac{\mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{1 \mathrm{cmol}_c}{\mathrm{cmol}}=2.00 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
Then add up all of the cations’ concentrations in cmolc/kg:
CEC = [Ca2+] + [Mg2+] + [K+] + [Na+] + [Al3+] + [H+]
= 9.25 cmolc/kg + 6.67 cmolc/kg + 1.36 cmolc/kg + 1.09 cmolc/kg + 1.33 cmolc/kg + 2.00 cmolc/kg
= 21.70 cmolc/kg
Approach 2: Take a Weighted Average of Known Colloids’ CECs
Soil particles are not created equal. Some have higher CEC’s than others. In particular, the vast majority of surface charge sites, and hence CEC, of most soils comes from clays and organic matter (and mostly from colloid-sized particles thereof). If we know the concentrations of the most common soil clays, as well as organic matter, we can then use these to estimate the CEC of the soil. To do this, we take a weighted average – like you would when calculating your grade.
The general formula is:
[latex]{CEC}_{soil}=sum_{i}^{n}{F_i{CEC}_i}[/latex]
Where:
- i = the individual soil component
- n = the number of individual soil components
- Fi = the fraction of the individual soil component
- CECi = the cation exchange capacity of the individual soil component
This means that for each soil component (clay or organic matter), you need to multiply the fraction that that component occupies of the whole soil (Fi) by that component’s CEC (CECi). Then, add up the products.
Because most of the CEC is coming from soil and clay, we can break this down a bit using another formula derived from the one above:
[latex]{CEC}_{soil}=F_{clay}{CEC}_{clay}+{F}_{OM}{CEC}_{OM}[/latex]
Where:
- CECsoil = the cation exchange capacity of the whole soil
- Fclay = the clay fraction, from 0 to 1
- CECclay = the CEC of all clay mineral particles, including multiple clay and colloid-sized minerals like phyllosilicates and oxides/hydroxides
- FOM = the organic matter fraction, from 0 to 1
- CECOM = the CEC of the organic matter
Each soil has a different suite of clays and/or organic matter contributing to its CEC. In order for this approach to work, the CECs of the clay and organic matter components must be known. Furthermore, since there are multiple clay minerals present in most soils, we must calculate CECclay before we can plug it into the formula. Assuming that only the 4 most common phyllosilicates are present in the clay fraction, then we can use the formula:
[latex]{CEC}_{clay}=F_{mica}{CEC}_{mica}+{F}_{smec}{CEC}_{smec}+F_{ver}CEC_{ver}+F_{kaol}CEC_{kaol}[/latex]
Or more simply:
[latex]{CEC}_{clay}=F_m{CEC}_{m}+{F}_s{CEC}_{s}+F_{v}CEC_{v}+F_{k}CEC_{k}[/latex]
Where:
-
Fmica= Fm = the fraction of mica out of total clay
-
CECmica = CECm = CEC of mica
-
Fsmec = Fs = the fraction of smectite out of total clay
- CECsmec = CECs = CEC of smectite
This is easiest to learn from examples.
Example Calculation with 4 Clays
A clay loam contains 4% soil organic matter, 30% sand, 25% silt, and 38% clay. The clay contains 30% mica, 40% smectite (specifically, montmorillonite), 20% vermiculite, and 10% kaolinite. The CEC’s of each colloid component are shown in this table:
| Kind of colloid | CEC (cmolc/kg) |
|---|---|
| Organic | 200 |
| Smectite (ex: Montmorillonite) | 100 |
| Vermiculite | 150 |
| Mica (ex: illite, muscovite, biotite) | 30 |
| Kaolinite | 8 |
First, notice that there is no sand or silt in the formulas, since these have negligible surface charges – so we can ignore the % sand and silt. Now, we have the CEC of the organic matter from the table, but no CEC of clay. So we need to substitute the CEC of clay formula into the general formula. First write the formulas:
[latex]{CEC}_{soil}=F_{clay}{CEC}_{clay}+{F}_{OM}{CEC}_{OM}[/latex]
[latex]{CEC}_{clay}=F_m{CEC}_{m}+{F}_s{CEC}_{s}+F_{v}CEC_{v}+F_{k}CEC_{k}[/latex]
Next, substitute the second equation into the first:
[latex]{CEC}_{soil}=F_{clay}(F_m{CEC}_{m}+{F}_s{CEC}_{s}+F_{v}CEC_{v}+F_{k}CEC_{k})+{F}_{OM}{CEC}_{OM}[/latex]
And then insert the CECs from the table, and the percentages from the prompt as fractions (divide by 100 to convert from % to fraction form):
[latex]CEC_{\text {soil }}[/latex]
[latex]=0.38\left( \small{0.3 \times 30} \frac{\mathrm{cmol}_c}{\mathrm{~kg}} \small{+0.4 \times 100} \frac{\mathrm{cmol}_c}{\mathrm{~kg}}\small{+0.2 \times 150} \frac{\mathrm{cmol}_c}{\mathrm{~kg}}+0.1 \times 8 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}\right)+0.04 \times 200 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
[latex]=34.7 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
Example Calculation with 2 Clays
Frequently, the clay component (“clay fraction”) is dominated by just 2 common phyllosilicates instead of 4. When a phyllosilicate or other clay-sized mineral isn’t mentioned in the prompt, just omit it from your formula or enter it as 0.
A clay loam contains:
- 2% SOM
- 34% sand
- 31% silt
- 33% clay
The clay contains 60% smectite (montmorillonite specifically) and 40% kaolinite.
Assume the CECs of each fraction are the same as in the previous question.
Start with the formula with the CECclay already substituted in:
[latex]{CEC}_{soil}=F_{clay}(F_m{CEC}_{m}+{F}_s{CEC}_{s}+F_{v}CEC_{v}+F_{k}CEC_{k})+{F}_{OM}{CEC}_{OM}[/latex]
Vermiculite and mica aren’t present, so we can delete them:
[latex]{CEC}_{soil}=F_{clay}({F}_s{CEC}_{s}+F_{k}CEC_{k})+{F}_{OM}{CEC}_{OM}[/latex]
If desired, we can distribute the Fclay:
[latex]{CEC}_{soil}=F_{clay}{F}_s{CEC}_{s}+F_{clay}F_{k}CEC_{k}+{F}_{OM}{CEC}_{OM}[/latex]
Now we plug in the numbers from the prompt (remember we can ignore sand and silt):
[latex]{CEC}_{soil}=0.33 \times 0.6 \times 100 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}+0.33 \times 0.4 \times 8 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}+0.02 \times 200 \frac{\mathrm{cmol}_c}{\mathrm{~kg}}[/latex]
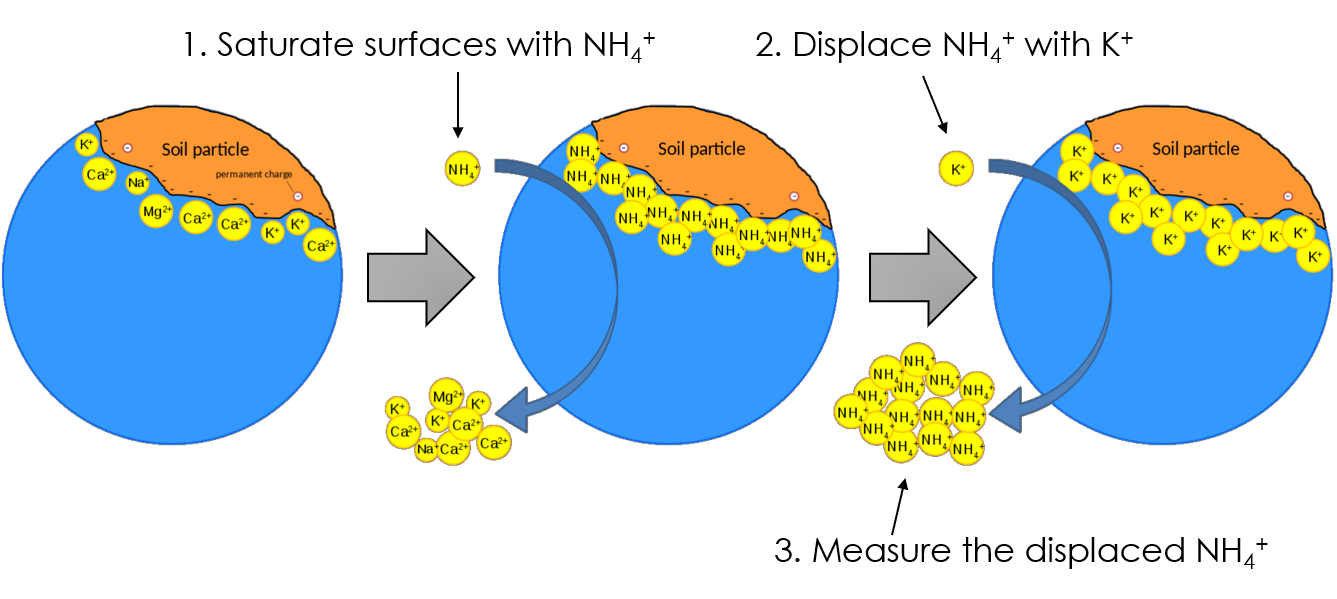
Approach 3: Cation Displacement
The cation displacement approach is generally regarded as the most robust for determining CEC. This is because it involves an actual exchange reaction, which ensures that you are definitely measuring cations exchanging – not cations that are found in soluble salts like CaSO4, and not cations that are adsorbed too tightly for exchange. This method is commonly used in introductory soil lab courses.
Method
Recall the steps of a typical cation displacement method:
- Replace all of the cations on the surface with NH4+
- Displace the NH4+ with copious K+
- Measure the displaced NH4+ in solution
- Convert units NH4+ was measured into cmolc/kg
The idea behind this method is to first flood the surfaces with an easily measurable cation like ammonium (NH4+), then “bump” that cation off the surface with excess concentrations of a displacing cation like K+, and finally measure the known cation. The approach gets its name from the displacement, the process by which the second cation “bumps” the first off surfaces and into solution. This method relies on a rule of cation exchange called “mass action” or “ratio law” where a very large quantity of a cation in solution will replace virtually all cations on the surface. In the end, the amount of that first cation (ex: NH4+) in solution is equal to the CEC.
This is typically measured by reacting the NH4+ with a reagent that changes color based on the concentration of NH4+. But, the units given aren’t normally the desired cmolc/kg, so we have to convert. That is the last step, the calculation. How we do this calculation depends on the units provided. See the example below for how to do the calculation when starting with the most common units, ppm (parts per million, equivalent to mg/L).
The final concentration of displaced ammonium (NH4+) in solution can be easily measured in ppm, which stands for parts per million, and is equal to mg/L. However, we need to convert to cmolc/kg. You might be wondering how we go from having volume (L) in the denominator to mass (kg). This has to do with the solution-to-soil ratio: the volume of solution that is reacted with a given mass of soil.
Let’s say that 20 mL of solution are reacted with 5g of soil, and the final concentration of NH4+ in solution is 60 ppm. Now let’s think about what we know, what we don’t know, and what we need to cancel out:
- We know that...
- ppm is the same as mg/L.
- the solution-to-soil ratio is 20 mL:5g soil, or 4 mL/g.
- there’s 1000 mL in a L
- there’s 1000 g in a kg
- theres 10 mmol in a cmol
- NH4+ has an absolute charge of 1, so it has 1 cmolc/cmol
- From the periodic table, N has a molar mass of 14 and H has a molar mass of 1. So, NH4+ has a molar mass of 14+4*1 = 18 g/mol = 18 mg/mmol.
- We want units of cmolc/kg
- We need to cancel out mg and L
Now we can arrange the conversion factors and ratios to convert the units we have (mg/L) to the units we want (cmolc/kg).
[latex]\frac{60 \mathrm{mg}}{\mathrm{L}} \times \frac{1 \mathrm{mmol}}{18 \mathrm{mg}} \times \frac{20 \mathrm{~mL}}{5 \mathrm{~g}} \times \frac{1000 \mathrm{~g}}{1 \mathrm{~kg}} \times \frac{1 \mathrm{~L}}{1000 \mathrm{~mL}} \times \frac{1 \mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{1 \mathrm{cmol}_c}{\mathrm{cmol}}[/latex]
[latex]= 1.33 \mathrm{ cmol}_{c}/ \mathrm{kg}[/latex]
Depending on the data available, CEC can be calculated 3 different ways:
- Sum of exchangeable cations
- Use when concentrations of exchangeable cations are available
- CEC = [Ca2+] + [Mg2+] + [K+] + [Na+] + [Al3+] + [H+]
- Weighted average of colloid CEC's
- Use when percentages or fractions of different colloids such as clays or organic matter are available. Also requires CEC's of each mineral or organic matter fraction, in units of cmolc per kg of colloid.
- General formula: [latex]{CEC}_{soil}=F_{clay}{CEC}_{clay}+{F}_{OM}{CEC}_{OM}[/latex]
- With specific colloids, abbreviated: [latex]{CEC}_{clay}=F_m{CEC}_{m}+{F}_s{CEC}_{s}+F_{v}CEC_{v}+F_{k}CEC_{k}[/latex]
- Quantification of displaced cations
- Use when the amount of a cation displaced back into the solution during cation exchange has been provided, such as NH4+
- This is a unit conversion, so it doesn't have a formula. For example, if 20 mL of solution are reacted with 5g of soil, and the final concentration of NH4+ in solution is 60 ppm, then we'd calculate:
- [latex]\frac{60 \mathrm{mg}}{\mathrm{L}} \times \frac{1 \mathrm{mmol}}{18 \mathrm{mg}} \times \frac{20 \mathrm{~mL}}{5 \mathrm{~g}} \times \frac{1000 \mathrm{~g}}{1 \mathrm{~kg}} \times \frac{1 \mathrm{~L}}{1000 \mathrm{~mL}} \times \frac{1 \mathrm{cmol}}{10 \mathrm{mmol}} \times \frac{1 \mathrm{cmol}_c}{\mathrm{cmol}}[/latex]

