35 Liming: Raising the pH of Acid Soils
Rivka Fidel
Upon completing this chapter, you should be able to:
- Define liming agent, agricultural lime, calcite, and dolomite
- Identify liming agents from a list of materials
- Explain how calcite and similar liming agents raise the pH of acid soil
- Estimate liming requirements from soil texture and pH using a graph
- Calculate a soil’s liming requirement using the acid saturation (%AS), CEC, volume (Vs), and bulk density (Db) of the soil
Liming
The pH of problematic acid soils (pH < 5.5) can be raised with alkaline materials called liming agents. The names “liming agent” and “agricultural lime” come from limestone, which is alkaline. Limestone is alkaline because it contains the base carbonate (CO32-), found in two common limestone minerals: calcite (CaCO3) and dolomite ((Ca,Mg)CO3). The most common liming agent is agricultural lime , a ground-up limestone product rich in calcite and/or dolomite. Besides adding agricultural lime to raise pH, other alkaline substances can be added, including quicklime (CaO), wood ash, and slag leftover from fossil fuel combustion.
Liming agents are all alkalis (bases containing alkali metals and/or alkaline earth metals) that work by accepting H+. For example, each mole of carbonate (CO32-) from calcite (CaCO3) can accept 2 moles of H+ in an acid-base reaction:
CaCO3(s) + 2H+(aq) ⇌ H2CO3 (aq) + Ca2+(aq)
The carbonic acid then turns into CO2 and H2O in a reaction called decarbonation (the reverse of carbonation):
H2CO3(aq) ⇌ ↑CO2(g) + H2O(l)
Notice how the CO2 is in the gas phase – this means it can easily escape the soil, decreasing the concentrations of the products in the soil and speeding up the reaction (aka “right shift”) via Le Chatelier’s Principle. Although largely beyond the scope of introductory soil science, briefly, Le Chatelier’s Principle states that a chemical reaction experiencing a change in reactant or product concentrations (or temperature or pressure changes) will shift oppositely from the change, back towards equilibrium.
Meanwhile, the Ca2+ ions do not directly raise the pH but rather help force more colloid-adsorbed H+ into the solution:
H+[Colloid]H+ + Ca2+(aq) ⇌ [Colloid]Ca2+ + 2H+(aq)
Notice how one Ca2+ from calcite replaces two H+ on the colloidal surfaces to maintain equivalent charges. Similarly, 3 Ca2+ ions from the solution replace 2 adsorbed Al3+:
2[Colloid]Al3+ + 3Ca2+(aq) ⇌ 3[Colloid]Ca2+ + 2Al3+(aq)
…and this Al3+ in solution then reacts with H2O to form H+ through hydrolysis:
Al3+(aq) + 3H2O(l) ⇌ Al(OH)3(s) + 3H+(aq)
Once in solution, the H+ can then react with more carbonate from the calcite (see the acid-base reaction above). Incidentally, adding liming agents like limestone help add Ca and Mg (Ca from calcite and dolomite found in limestone, and Mg from dolomite only). This is beneficial because, as mentioned earlier, problematic acid soils are commonly deficient in these nutrients.
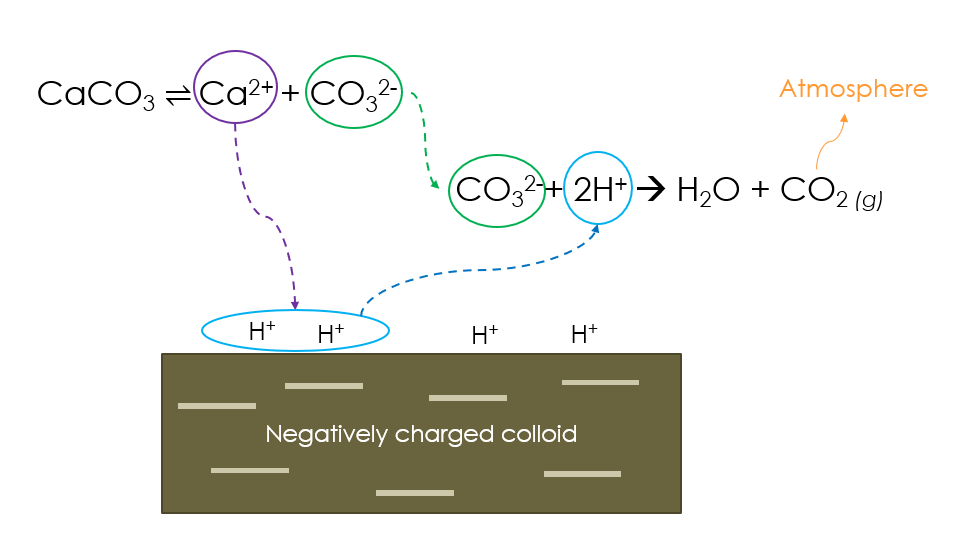
Thus calcite and other liming agents raise the pH of acid soils by accepting H+ (i.e. acting as bases), and the presence of alkali and/or alkaline earth metals like Ca2+ therein both augment their efficiency and provide plant nutrients.
For optimal results, soil in most cases should be limed to pH ~6.5. Doing so requires neutralizing not only the acid cations (H+ and Al3+) in solution but also those adsorbed to surfaces (acid cations inside particles do not require neutralization because they affect pH very slowly, over the course of many years). Liming to pH ~6.5 instead of ~7 reduces the risk of overshooting and causing the soil to become alkaline (pH > 7). Check out the next chapter on alkaline soils for more information about how high pH can be bad for plant growth.


Estimating the Limestone Requirement Using pH and Soil Texture
The amount of agricultural lime needed to raise the pH of a soil is known as the limestone requirement, and can be calculated based on its pH and CEC. However, sometimes CEC isn’t known. In this case, we can estimate the limestone requirement based on initial soil pH and texture instead using the below graph.
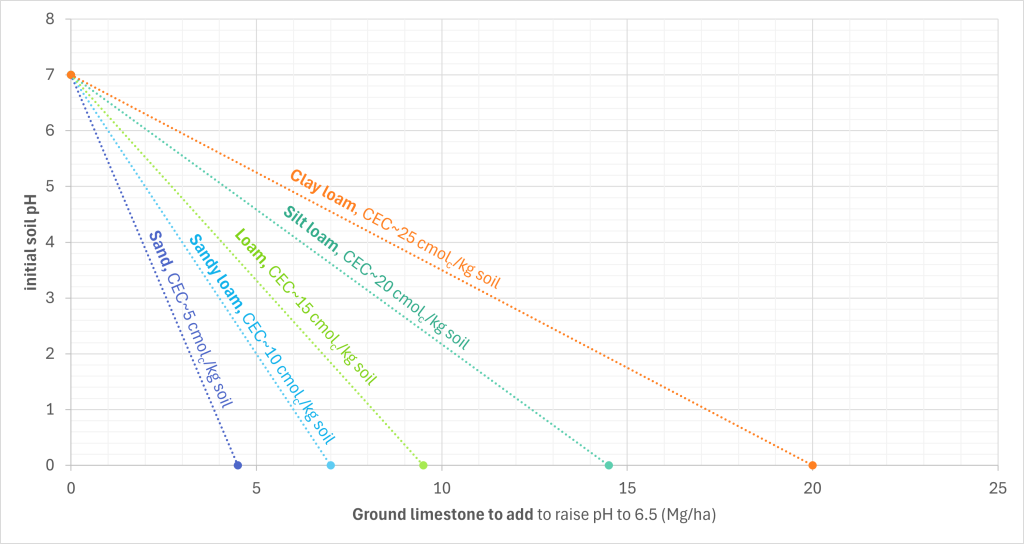
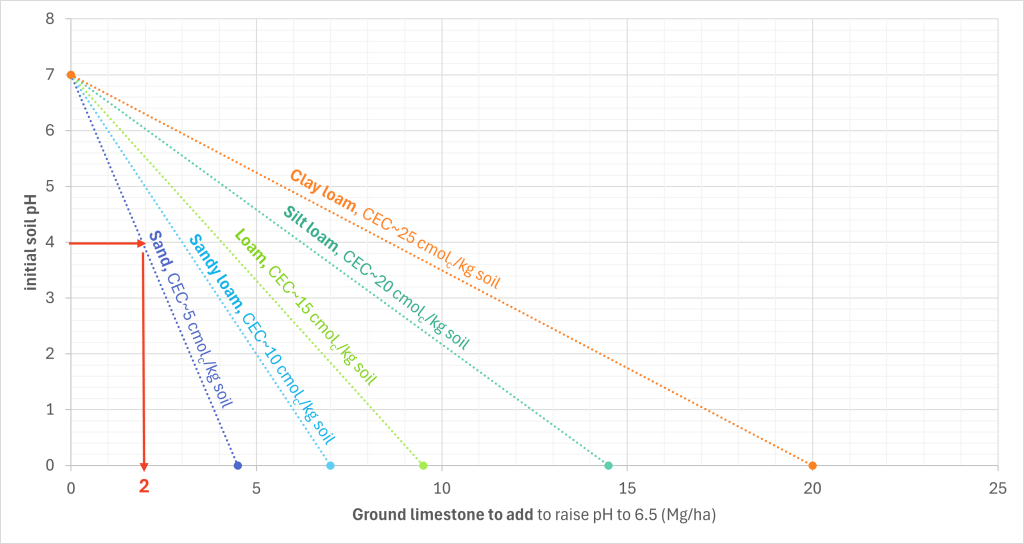
To use the graph, find the initial soil pH on the y-axis and draw a line to the curve pertaining to your soil’s texture. Then, we draw another line straight down, parallel to the y-axis. Then when this vertical line reaches the x-axis, the x-axis value is the limestone requirement.
When a soil with a sand texture has a pH of 4, we find 4 on the y-axis, draw a horizontal line to the sand curve, and then draw a vertical line straight down. This intercepts the x-axis at 2, so we know that 2 Mg/ha is the liming requirement.
Using Acid Saturation to Calculate Liming Requirements
Beyond estimation, how can you calculate liming requirements more precisely?
To lime an acid soil, you must neutralize both the acid cations in solution (called active acidity) exchangeable acid cations adsorbed to surfaces (called exchangeable acidity). In an acidic soil, the amount of exchangeable acid cations (mainly adsorbed to colloids) far outweighs the amount of acid cations in solution. Therefore, if we know the CEC and the acid saturation (AS) of a soil, we can estimate the liming requirement following the steps below.
- First, calculate the exchangeable acidity by multiplying the acid saturation (as a fraction) by the cation exchange capacity:
EA = AS*CEC
- Then, calculate the mass of the soil:
Ms = Db*Vs
- Next, multiply the mass of the soil by the exchangeable acidity in cmolc/kg to get the total exchangeable acid cations in cmolc:
TEA = EA*Ms
- Then, convert the units to kg of CaCO3 to get the calcite requirement (CR):
CR = TEA*(100 g CaCO3/mol)
- Finally, most agricultural liming products do not have 100% effectiveness, or effective calcium carbonate equivalent (ECCE). ECCE measures how much acid agricultural liming products can neutralize relative to pure, powdered calcite. The higher ECCE, the more effective the liming agent is, and the less needs to be applied; the lower the ECCE, the less effective the liming agent is, and the more needs to be applied. So, when ECCE < 100% (as is almost always the case), we have to calculate the liming requirement (LR), by dividing the calcite requirement (CR) by the ECCE:
LR = CR ÷ ECCE
This calculation assumes that the amount of acid cations in solution is negligible compared with the amount on exchange sites. Refer to the examples below for how to adjust the steps based on the volume of soil, provided units, and desired output units.
For a raised bed 1 m3 in volume, containing a soil with bulk density of 1.3 g/cm3, a CEC of 20 cmolc/kg, and an acid saturation of 60%, how much agricultural lime is needed? Assume the agricultural lime (ground up limestone) has an ECCE of 80% (i.e. 80% of the limestone is calcite that will dissolve in the near term).
- First, calculate the exchangeable acidity by multiplying the acid saturation (as a fraction) by the cation exchange capacity:
EA = AS*CEC
= (20 cmolc/kg * 0.6 cmolc of acid cations/total cmolc )
= 12 cmolc acid cations/kg
- Then, calculate the mass of the soil (notice the conversion factors used to convert g to kg and m3 to cm3)::
Ms = Db*Vs
=(1.3 g/cm3 ÷ 1000 g/kg)*(1 m3 * 1003 cm3/m3)
=1300 kg
- Next, multiply the mass of the soil by the exchangeable acidity in cmolc/kg to get the total exchangeable acid cations in cmolc:
TEA = EA*Ms
= 12 cmolc acid cations/kg * 1300 kg
= 15600 cmolc acid cations - Then, convert the units to kg of CaCO3 to get the calcite requirement (CR):
CR = TEA*(0.0005 kg CaCO3/cmolc)
= 15600 cmolc*(0.0005 kg CaCO3/cmolc)
=7.8 kg
- Finally, calculate the liming requirement (LR), by dividing the calcite requirement (CR) by the ECCE. The ECCE needs to be written as a fraction, 80% becomes 0.8:
LR = CR ÷ ECCE
=7.8 kg ÷ 0.8
= 9.75 kg
Notice how more lime is needed when the liming agent is just 80% effective. If it were 100% effective, we could have just added 7.8 kg of agricultural lime – but because it was just 80% effective, we have to add 9.75 kg.
Now, imagine you need to neutralize the acidity of a whole acre of soil, 1 foot deep. If soil still has a bulk density of 1.2 g/cm3, a CEC of 15 cmolc/kg, and an acid saturation of 70%, how much agricultural lime is needed? Assume the agricultural lime (ground up limestone) has an ECCE of 90% (i.e. 90% of the limestone is calcite that will dissolve in the near term).
- First, calculate the exchangeable acidity by multiplying the acid saturation (as a fraction) by the cation exchange capacity:
EA = AS*CEC
= (15 cmolc/kg * 0.7 cmolc of acid cations/total cmolc )
= 10.5 cmolc acid cations/kg
- Then, calculate the mass of the soil. One acre-foot is equal to 1233 cubic meters, so:
Ms = Db*Vs
=(1.2 g/cm3 ÷ 1000 g/kg)*(1233 m3 * 1003 cm3/m3)
=1479600 kg
- Next, multiply the mass of the soil by the exchangeable acidity in cmolc/kg to get the total exchangeable acid cations in cmolc:
TEA = EA*Ms
= 10.5 cmolc acid cations/kg * 1,479,600 kg
= 15,535,800 cmolc acid cations - Then, convert the units to kg of CaCO3 to get the calcite requirement (CR):
CR = TEA*(0.0005 kg CaCO3/cmolc)
= 15,535,800 cmolc*(0.0005 kg CaCO3/cmolc)
=7,768 kg
- Finally, calculate the liming requirement (LR), by dividing the calcite requirement (CR) by the ECCE. The ECCE needs to be written as a fraction, 90% becomes 0.9:
LR = CR ÷ ECCE
=7,768 kg ÷ 0.9
= 8,631 kg
Optionally, if metric tons are preferred, we can divide by 1000 to get metric tons (denoted as Megagrams, Mg):
8,631kg ÷ 1000 kg/Mg
= 8.631 Mg
If short tons are desired instead, we can divide kilograms by 907.2 kg/ton:
8,631kg ÷ 907.2 kg/ton
= 9.51 tons
The calculated amount, no matter whether it’s in metric tons or short tons, is the amount needed to neutralized the exchangeable acidity only in the top 1 ft only of soil, spread over 1 acre. We’re assuming here that:
- the movement of acid cations upwards from below the 1 ft depth will be negligible over the course of the growing season
- the amount of acid cations in solution is negligible compared with the amount on exchange sites
You can find three methods for determining liming requirements, including one nearly identical to this one, in the Soil & Plant Growth Laboratory Manual – Exercise 6A. Note that some definitions of ECCE differ from what’s used here. In this chapter, ECCE is a percent or fraction, but other definitions use absolute units instead.
Benefits of Liming Acid Soils
From the soil acidity impacts outlined in the acid soils chapter, we can infer that raising problematic acid soil pH to ~6-6.5 would normally result in:
- Reduced aluminum toxicity due to decreased solubility of Al3+
- Reduced risk of transition metal micronutrient toxicity (Fe2+, Mn2+, Cu2+ and Zn2+)
- Increased cationic macronutrients: Ca2+, Mg2+, and K+, because:
- Calcite directly adds Ca2+, and dolomite adds both Ca2+ & Mg2+
- Increasing pH increases CEC, enhancing cation retention
- Increased plant-available P due to less with Al and Fe precipitating with PO43-
- Increased activity of potentially beneficial microbes, as well as earthworms
Remember to avoid overshooting – these benefits will be overshadowed by alkaline soil problems if a pH of 7 is exceeded. That is why we aim for a pH of 6 to 6.5.
- Soil acidity can be alleviated by adding liming agents to raise the pH. The most common is agricultural lime.
- Agricultural lime = ground-up limestone, containing the alkaline, carbonate minerals calcite (CaCO3) and/or dolomite ((Ca,Mg)CO3)
- Target a pH of ~6.5 for optimal results
- The liming requirement can be estimated from soil texture and pH using a graph
- The graph provided here is only for humid, temperate regions.
- For other regions, you must first determine the CEC to use the graph (or the calculation below).
- The liming requirement can be calculated from the acid saturation (%AS), CEC, volume (Vs), and bulk density (Db) of the soil using these equations:
EA = AS*CEC
Ms = Db*Vs
TEA = EA*Ms
CR = TEA*(100 g CaCO3/mol)
LR = CR ÷ ECCE

