36 Salt-affected Soils: Salinity and Sodicity
Rivka Fidel
- Define: salinity, sodicity, saline soil, sodic soil, saline-sodic soil, EC, ESP, SAR
- Calculate: ESP
- Explain what type of climates would you expect to find acid, alkaline and salt-affected soils in and why
- Describe why plants have trouble growing in saline, saline-sodic, and sodic soils
- Compare and contrast approaches for reclaiming saline, saline-sodic, and sodic soils
Salt-affected soils
Salts are defined as ionic compounds containing a cation and an anion. The types of salts accumulating in salt-affected soils specifically tend to contain an alkali metal or alkaline earth metal cation bonded to a nonmetal anion. In order to translocate downwards through the soil, the salts need to be fairly soluble so that they can first dissolve in the topsoil, then move down and precipitate in the subsoil. Here are some example salt dissolution reactions for common salts found in soil:
- Calcium carbonate: CaCO3 ⇌ Ca2+ + CO32-
- Sodium chloride (table salt): NaCl ⇌ Na+ + Cl-
- Gypsum: CaSO4 •2H2O ⇌ Ca2+ + SO42- + H2O
- Potassium nitrate (fertilizer): KNO3 ⇌ K+ + NO3-
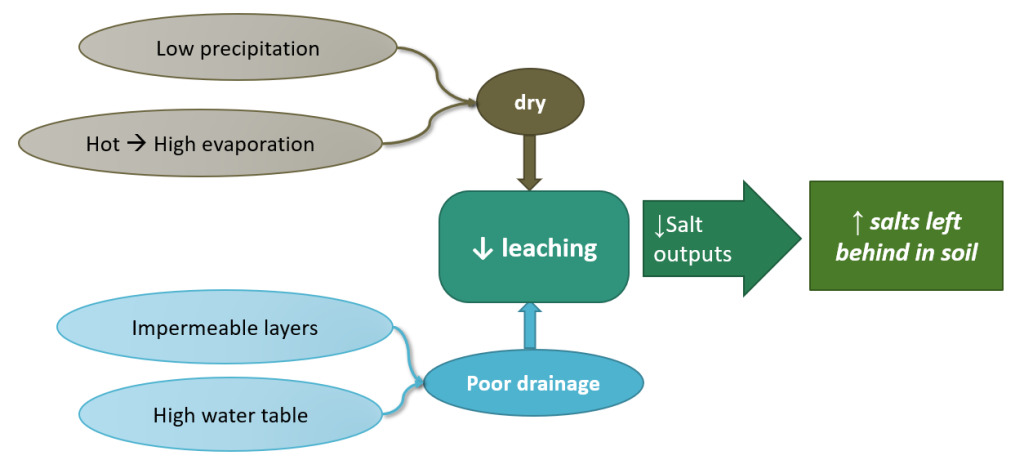
Salt-affected soils are soils with excessive total salt concentrations or imbalances among salts such that they inhibit plant growth. These soils are most commonly found in semi-arid to arid regions, because in dry climates the low precipitation leads to low leaching of salts, and instead salts accumulate in the soil profile. Salt accumulations are therefore common among Aridisols, especially in B horizons. Salt-affected soils can also be found in areas with poor drainage such as closed basins. Closed basins are areas with a bowl-like shape where the water cannot flow out to a nearby stream, but instead collects in the middle where much of it evaporates, leaving behind salts. Such areas generally also have high clay content and poor drainage due to the downhill movement of clays, preventing salts from leaching out to the groundwater table and further promoting salt accumulation.
In the environment where a soil is developing, salts originate from weathering of minerals. From there they dissolve, and move with the flow of water through soil, groundwater, and/or streams. Ultimately these salts end up in the ocean. If groundwater or stream water are used for irrigation, this adds salt back into the soil; and if much of this evaporates due to hot, dry conditions, salts can accumulate in irrigated soils. Such salts in irrigation water pose common challenge farmers in arid regions have faced for millennia, from Mesopotamia to the modern Western US and Middle East. Furthermore, coastal regions face a threat of incoming salts from ocean water, in the form of salty sea spray, saltwater intrusion into fresh groundwater, and, in more recent decades, sea level rise. Such ocean-derived salts can quickly turn healthy coastal soils into salt-affected soils.
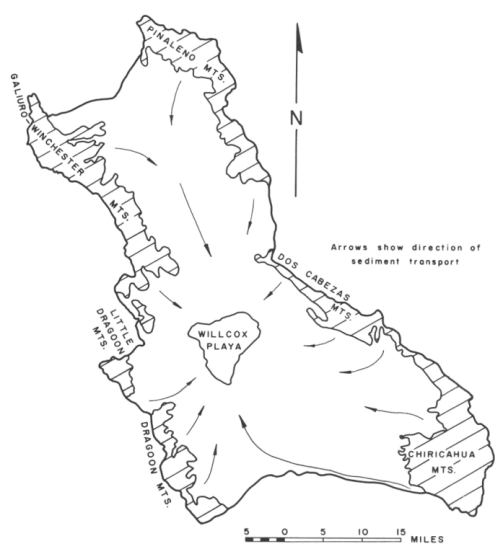
An example of an extremely salt-affected soil is found at Willcox Playa, in Cochise County, Arizona. Willcox Playa is located in a closed basin, meaning it's a the lowest point of a bowl-like area surrounded by hills and mountains. As such the water from the rest of the landscape flows towards the playa, with no other outlet. Thousands of years ago - in the late Pleistocene Epoch, when the local climate was wetter - the inflow of water once formed Lake Cochise, and when the climate became drier ~11,000 years ago, the lake water evaporated, leaving behind salts. Over time the water continued to flow towards the playa, carrying with it salts from higher in the landscape, and evaporating - leaving behind even more salts. The subsoil is furthermore very clayey, reducing leaching of salts out of the soil through percolation and drainage. The soil hence grew ever more salty over time, until it could no longer support most plants.


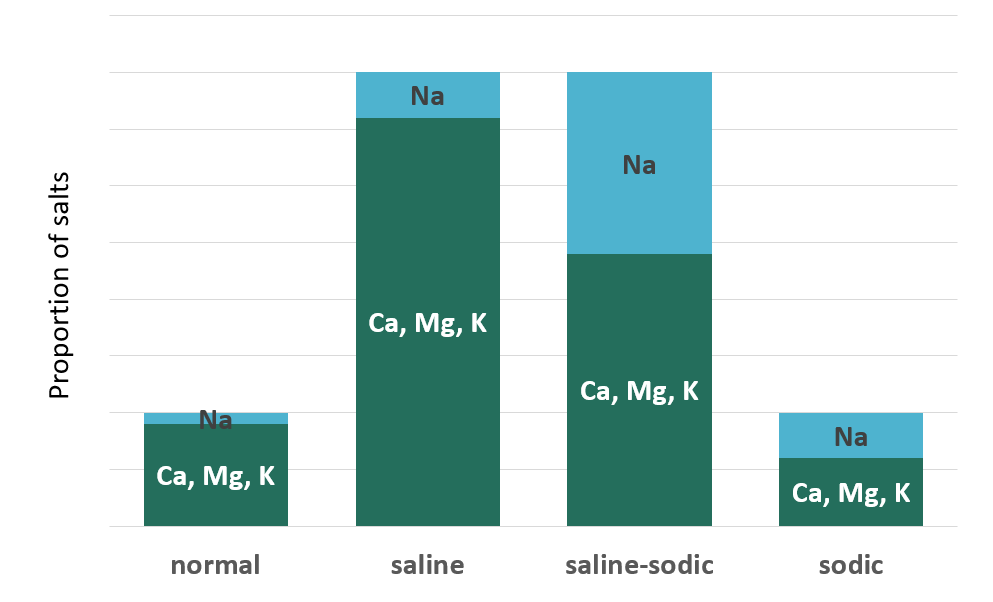
Saline and Sodic Soils
There are three types of salt-affected soils: saline, sodic and saline-sodic. These are classified based on their salinity (total salt content) and sodicity (the proportion of sodium).
Salinity
Measuring Salinity
Total salt concentrations can be quickly measured using electrical conductivity (EC). Salts generally increase the EC of water because the charges of ions (both cations and anions) in salts help carry electrical current through water, increasing its electrical conductivity. Thus, the higher a soil’s EC, the saltier it is, and the greater its salinity. Soils with EC’s greater than 4 dS/m are considered saline soils.
Salinity testing via the saturated paste method
Saline Soils
In saline soils, typical plants may experience osmotic stress. Osmotic stress occurs when a high total salt concentration lowers osmotic potential so low that plants have trouble getting water. As you may recall from the soil water chapter, water flows from high to low water potential. Also recall that as salt concentration increases, osmotic potential decreases. So, if the soil water potential is too low because the soil is too salty, plants can’t get water. In other words, the salts may literally suck water out of the plants! Some plants, called halophytes, have evolved to keep their water potentials low enough to grow in saline soils. These plants grow in naturally saline soils found in coastal areas and closed basins. Generally though, typical plants struggle to get water in saline soils.
More About Osmotic Stress
Watch this video for more information: Unavailability of water in saline soils
Sodicity
Measuring Sodicity
Sodicity is measured using exchangeable sodium percentage (ESP), the fraction of sodium out of the total exchangeable ions:
[latex]ESP = \frac{\textit{exchangeable } Na^+}{CEC} = \frac{[Na^+]}{[Ca^{2+}] + [Mg^{2+}] + [K^+] + [Na^+]}[/latex]
ESP is calculated from cmolc/kg of Na, and the CEC in cmolc/kg. Here the CEC can be estimated as the sum of all of the base cations, also in cmolc/kg.
Sodicity of soil can alternatively be measured using Sodium Adsorption Ration (SAR), which is calculated from concentrations of soluble cations in a soil extract or in water, in units of mmol/L:
[latex]SAR = \frac{[Na^+]}{\sqrt{0.5[Ca^{2+}] + 0.5[Mg^{2+}]}}[/latex]
The higher the ESP or SAR of a soil, the greater its sodicity. Soils with an ESP > 15% or a SAR > 13 and EC < 4 are considered sodic soils. That means a soil must have both a high proportion of sodium relative to other nonacid cations and low total salts to be sodic.
Sodic Soils
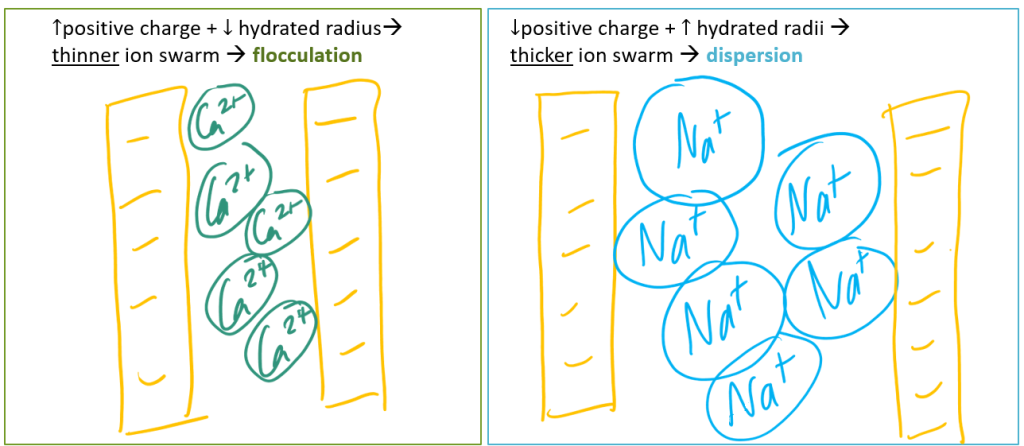
Compared with the other nonacid cations, Na+ is special in that it both degrades soil structure and increases soil alkalinity. Thus, excess sodicity degrades the soil both chemically and physically.
When present in excessive amounts (>15% on a molar basis), it causes dispersion of clays and a subsequent decline in soil aggregation. Dispersion is when clay particles, instead of sticking together, come apart. This is a problem because clays are important for aggregation or “clumping” of soil, which improves soil physical properties such as infiltration, drainage, and aeration. Once dispersed, clays can also flow through the soil and clog up pores, further reducing infiltration and drainage. When clay dispersion occurs on the surface of the soil, creating a sort of clayey mud puddle, the clays harden as they dry, forming a hard crust in a phenomenon called crusting. In addition to reducing infiltration, these crusts make it difficult for young plants to break through the soil and reach sunlight.

Then, sodium increases soil pH to above 8.5, which inhibits the growth of average plants. Sodium increases the solubility of carbonates (CO32- -bearing compounds), because sodium carbonate (Na2CO3, also called super washing soda) is much more soluble than the more common calcium carbonate (CaCO3, aka calcite). As a result, soils rich in Ca2+ have low CO32- solubility, and so CaCO3-rich soils typically have pHs ranging from 7.6-8.4. However, excessive Na+ relative to other nonacid cations helps the carbonate (CO32-) dissolve much better than it would in a soil with sufficient Ca2+ or Mg2+, raising the pH to 8.5 and above.
Why do sodic soils undergo dispersion?
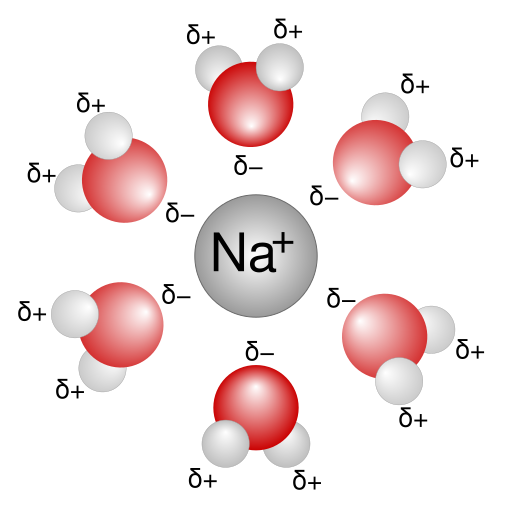
The “sod” in sodic stands for “sodium.” Sodium (Na) is special among the four main nonacid soil cations (Na, K, Mg and Ca) in that it has the largest hydrated radius relative to its charge. The hydrated radius is the distance from an ion to the edge of the waters surrounding it.
The large hydrated radius of Na acts like a "bumper" surrounding each sodium ion, preventing it from getting close to negatively charged surfaces like those found on phyllosilicate clays. When many Na ions are present, they create a thick ion swarm that keeps clay particles dispersed. This causes the soil to lose much of its aggregation and structure.
This video shows how dispersion works, though the cation's hydrated radii aren't shown (only charges): Dispersion of clay plates by sodium in soil
Saline-Sodic Soils
What about soils that have too much total salts and too much sodium? These soils, which have ESP > 15% (or SAR > 13) and EC > 4 are called saline-sodic soils. Interestingly, these soils in fact have better structure than sodic soils. Why? Because the presence of excess total salts (aka salinity) actually negates the effects of excess sodium (aka sodicity). This occurs because as salinity (and EC) increase, flocculation and therefore aggregation increase. As a result, saline-sodic soils are more fertile than sodic soils, but less fertile than saline soils.
More About the Sodium Adsorption Ratio (SAR)
Watch this video for more information: What is the sodium adsorption ratio?
Remediating Salt-Affected Soils
Many soils are naturally saline and/or sodic, and still support the growth of native salt-adapted plants. In this case, the ecosystem can remain healthy without intervention. However, remediation becomes necessary when a soil has become saline or sodic as a result of poor management, or when a landowner needs to grow crops on a naturally salt-affected soil. Irrigated farmland in dry regions commonly becomes saline and/or sodic over time due to the presence of salts or predominance of sodium in irrigation water. It is ideal to prevent the development of salinity or sodicity in the first place by using low EC, low SAR irrigation water (i.e. water low in total salts and in sodium).
When soils do need remediation, saline soils need different treatment from sodic soils. Saline soils simply require irrigation with excess clean water having a low EC. This washes excess salt from the upper soil layers where plants grow down to the groundwater permeating lower soil layers or fractured rock below.
Sodic soils, however, first require application of gypsum (CaSO4 ●2H2O) before adding excess clean irrigation water to remediate them. The purpose of the gypsum is to replace the Na+ adsorbed to soil colloid surfaces with Ca2+:
Na+[Colloid] Na++ CaSO4(aq)⇌Ca2+ [Colloid] + Na2SO4 (aq)
…where [Colloid] stands for any negatively charged colloid particle. Note that calcite (CaCO3) should never be added to sodic soils. This is because unlike gypsum, calcite contains CO32-; this base would further increase the pH of sodic soils, which are already too alkaline. The SO42- in gypsum, on the other hand, does not increase the pH because, as the conjugate anion of a strong acid, it cannot accept H+ in soil.
In addition to Ca2+, H+ can be used to remediate sodic soils. H+ can be added in the form of sulfuric acid (H2SO4), which is a strong acid that completely dissociates into 2H+ and SO42- in water (technically HSO4- is not a strong acid, but it’s strong enough that it will completely dissociate at typical soil pH’s ranging from 4 to 10). The H+ both lowers the pH and replaces Na+ adsorbed to colloids.
This reaction shows how H+ replaces adsorbed Na+:
Na+2[Colloid] + H2SO4(aq) ⇌ H2+[Colloid] + Na2SO4 (aq)
…and this reaction shows how H+ neutralizes carbonates:
H2SO4(aq) + Na2CO3(aq)⇌CO2(g) + Na2SO4(aq) + H2O

Videos Showing the Effect of Gypsum on Sodic and Saline-Sodic Soils
Watch this video for more information: Visualizing soil properties: Dispersion and flocculation
For more information about how to manage salt-affected soils, see Managing Salt-Affected Soils for Crop Production.
Summary Table for Acidity, Alkalinity, Salinity and Sodicity
This table summarizes the properties and remediation techniques for "problem soils" we've explored in the last few chapters.
|
|
Acid soil | Alkaline soil | Saline soil | Saline-Sodic soil | Sodic soil |
|---|---|---|---|---|---|
|
Defining feature |
Lots of H+ | Lots of OH- | Too much total salt | Too much total salt and Na | Too much Na relative to other salts |
|
Identified by |
pH < 7 (only a problem if pH <5.5) |
pH > 7 (bigger problem if pH > 8.5) |
EC > 4 ESP < 15% SAR < 13 pH 7-8.5 |
EC > 4 ESP >15% SAR > 13 pH > 7 |
EC < 4 ESP > 15% SAR > 13 pH > 8.5 |
|
Soil structure |
Good | Good | Good | Adequate to good | Poor |
|
Environmental conditions |
Heavy leaching conditions (Spodosols, Ultisols + Oxisols) |
Dry, arid regions Limited leaching conditions |
|||
|
Remediation |
Liming (add calcite, i.e. CaCO3) |
Usually too expensive to add enough acid | Add water (low EC, low SAR) |
Gypsum (CaSO4), then water (low SAR water is best) |
|
Note: A soil suitable for growing average crops (not in the table) would have a pH between 6-7, EC < 4 dS/m, and ESP < 15%.
Salt-affected soils form in dry conditions. There are 3 types:
- Saline soils
- Too much total salt
- EC > 4 dS/m & ESP < 5%
- Cause osmotic stress
- Good structure, infiltration, and drainage
- To remediate: add high quality (low-salt, low-sodium) water
- Sodic soils
- Too much sodium relative to other exchangeable cations
- EC < 4 dS/m & ESP > 15%
- pH > 8.5
- Poor structure, infiltration and drainage
- To remediate: add gypsum, then water low in salts/sodium
- Saline-sodic soils
- High total salts and sodium relative to other exchangeable cations
- EC > 4 dS/m & ESP > 15%
- Adequate to good structure, infiltration, and drainage
- To remediate: add gypsum, then high-quality water low in salts/sodium



