21 Soil Redox Processes
Rivka Fidel
- Identify features of reduction-oxidation processes in soil
- Explain significance of redox features
- Predict the order in which microbes will use different oxidizing agents based on the redox ladder
Importance of the Soil Redox Process

Reduction and oxidation (redox) reactions affect numerous soil processes, ultimately influencing nutrient availability and mobility, microbial activity, soil pH, and even soil color.
Redox processes alter soil’s appearance in ways that help us interpret soil formation conditions. Reduction and oxidation features (redox features, or redoximorphic features) are indicators of important soil conditions, particularly oxygen status. Even in drought conditions, soil redox features can be used to avoid potentially costly issues, like basement or septic system placement in an area with regularly saturated conditions.
Redox Reaction Basics
Reduction reactions occur when an element gains electrons from another element, reducing their charge (making the charge more negative). Oxidation reactions occur when an element loses electrons. To help you remember, you can use the mnemonic “LEO the lion says GER“, standing for:
- LEO: Loss of Electrons is Oxidation
- GER: Gain of Electrons is Reduction
Soil scientists apply redox concepts from chemistry to help understand and predict redox processes in soils. Here are some videos explaining redox reactions and how to interpret them at an introductory level (not specific to soil science):
Note that you would not expect to find many of the chemicals in these videos, like F2 or Mg0, in soil.
Some of the most common elements to undergo redox reactions in soil are iron (Fe) and manganese (Mn). They tend to lose their electrons to elements like O in oxygen gas (O2) and N in nitrate (NO3–).
Both O and N – nonmetal elements that frequently gain electrons, and become “reduced” – are what’s called oxidizing agents, because they help oxidize other elements. Of these, oxygen is the best oxidizing agent found in nature. That is why organisms that use oxygen for respiration are so common.
Metals like Fe and Mn, however, tend to act as reducing agents, because they help reduce the O and N.
You may have wondered why metals rust, or why you can’t find tiny bits of iron or copper ore lying around in the soil. “Rusting” phenomena are redox reactions! For example, the iron found in iron ore and cast iron pots is Fe0. Fe0 readily donates electrons, as shown in this half reaction:
Fe0 ↔ Fe2+ + 2e–
Notice how the Fe0 gave up 2 electrons, which appear in the products. In giving up electrons, the Fe was oxidized, and developed a 2+ charge. Fe2+ can precipitate with OH- and form green rusts. Or, it can react again, like this:
Fe2+ ↔ Fe3+ + e-
Now, you may be wondering where these electrons are going. In nature, oxidation reactions are always paired with reduction reactions, because the electrons must always go somewhere. Half reactions only show half the story! In well-aerated soils, the most common oxidizing agent is O2 gas. It is reduced via this half reaction:
O2 + 4e- ↔ 2O2-
Here, the two oxygens in oxygen gas have accepted 2 electrons each, for a total of 4 electrons.
If we double the first half reaction with iron, and then add it to the one with oxygen, we can write a balanced reaction like this:
2Fe0 + O2 ↔ Fe2+ + O2-
The iron has donated 2 electrons per atom, or 4 total, to the oxygen gas. The oxygen gas develops a -2 charge, and the iron develops a +2 charge. Then, in the presence of water, Fe reacts with water to form green rust:
Fe2+ + 2H2O ↔ Fe(OH)2
Any Fe2+ in solution can alternatively react again with oxygen like this:
2Fe2+ + O2 + ↔ Fe3+ + 2O2-

Reduction Features
Reduced conditions, shown by the grey colors, indicate a lack of oxygen in that soil area. This is frequently associated with saturated conditions, but additional factors can contribute. Even during a drought, redox colors developed due to regular saturation will still be present, making soil color an important indicator of expected wetness conditions.
Reduced iron is mobile, meaning it can move in the soil solution until it comes in contact with oxygen. Other elements such as nitrogen, manganese, and sulfur can also become reduced. These elements are considered “redox active”.

Changes in Redox Reactions after Flooding: The Redox Ladder
To understand how wetlands function, and how flooding affects non-wetland soils, we need to examine microbes that grow in saturated (waterlogged) conditions and how they respire. All respiration reactions require an electron acceptor, a compound or element also known as an oxidizing agent that can accept electrons at the end of the electron transport chain. Thus far we’ve only discussed aerobes, or aerobic microorganisms that use O2 for respiration. However, following flooding, aerobes quickly use up dissolved O2. Some microorganisms can only use O2 (obligate aerobes), and these quickly die – but other microorganisms called anaerobes can use other electron acceptors (oxidizing agents) such as NO3–.
However, these alternative electron acceptors come with a drawback: they do not yield as much energy as oxygen. When we arrange common electron acceptors from those that yield the most energy to ones that yield the least, we end up with a diagram called a redox ladder, shown below. Each stair step of the redox ladder represents a half reaction performed by different microbes (mostly bacteria and archaea). On the top of each stair step is reactant, so the electron acceptor prior to accepting electron(s), aka the element in it’s “oxidized form”; on the bottom of each stair step is the product, including the same element after accepting electrons, aka the element in its “reduced form.”
As you go down the redox ladder, each respiration reaction yields less energy. For example, the first step shows O2, the most effective electron acceptor, on the top. On the bottom of the stair step is H2O, which includes O2-. This denotes that O0 in O2 gained was reduced to O2- in H2O because it gained 2 electrons. On the next step, NO3– is on the top of the stair step, and N2 is on the bottom. This means that the N5+ in NO3– accepted electrons, and was reduced to N0 in N2. Because NO3– appears below O2, we know that using it as an electron acceptor yields less energy.
Because of the decline in energy yield going down the redox ladder, the microbes performing each successive step in the redox ladder grow slower than those above. So, aerobes grow the fastest, then denitrifiers (a type of NO3–-reducers), then manganese reducers, iron reducers, and so forth. This is why the microbial community collectively will use the best electron acceptor available, i.e. the oxidizing agent listed highest in the ladder. More about these and other soil microbes can be found in Part X on Soil Biology.
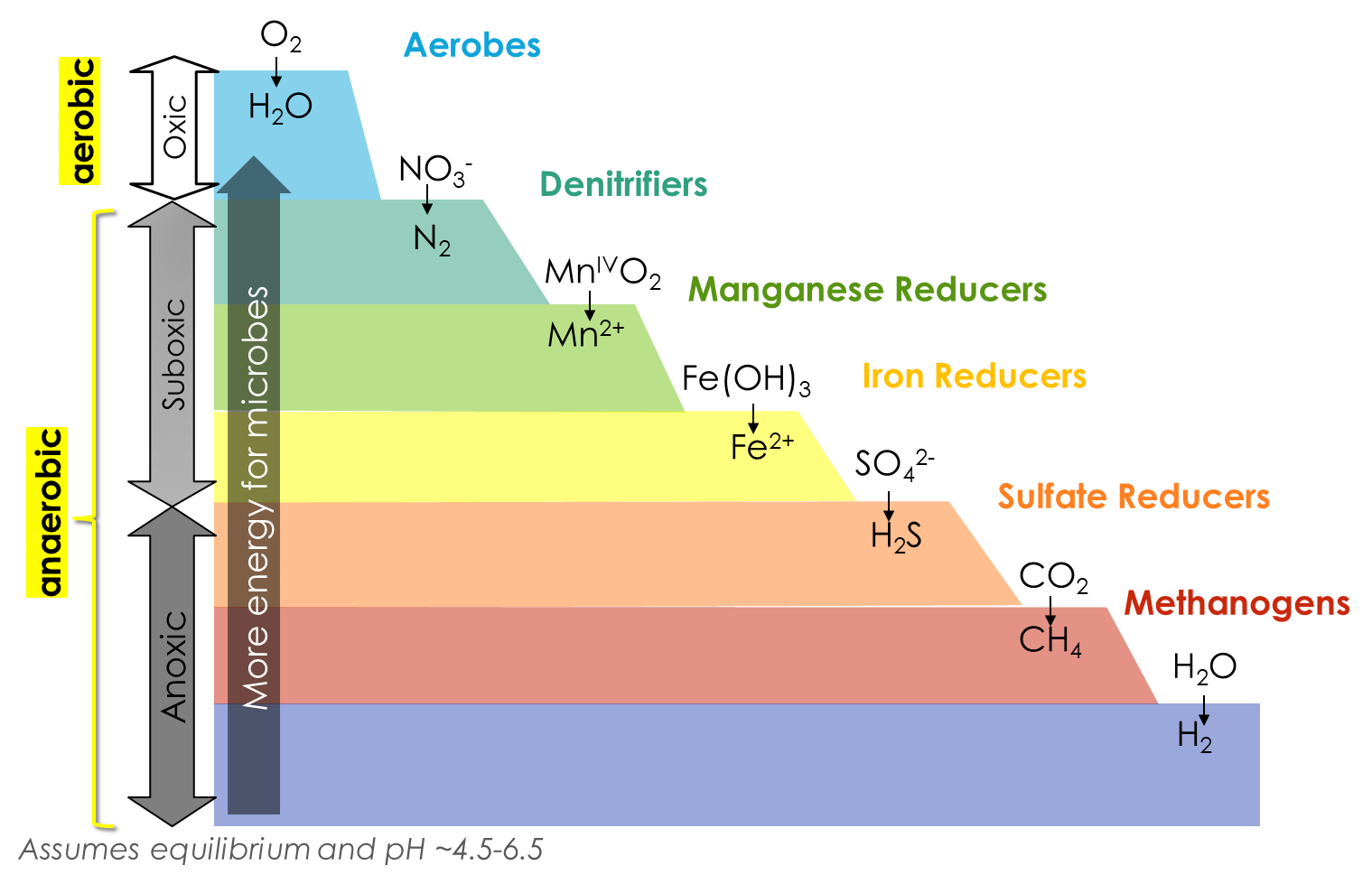
In the graphic above, the top of each step on the ladder shows the oxidizing agents prior to accepting electrons (“oxidized forms”), and the bottom of each stair step shows the same compounds after accepting electrons (“reduced forms”). For example, Mn4+ in MnO2 at the top of the 3rd step is the 3rd best oxidizing agent in the ladder, and when it accepts 2 electrons, it becomes Mn2+, found at the bottom of the same step. This reduction half-reaction is performed by microbes called manganese reducers, as indicated by the label to the right of the stair step. Also notice that the soil is considered aerobic (or oxic) when sufficient O2 gas is available for respiration, and when this O2 runs out, the soil becomes anaerobic. Among anaerobic conditions, we can further divide the soil status into suboxic and then anoxic depending on which oxidizing agents are available.
Why do different electron acceptors yield different amounts of energy?
You might be wondering why exactly the different electron acceptors (oxidizing agents) in the redox ladder yield different amounts of energy, and why this decreases going down the ladder. It comes down to two concepts from chemistry: redox potential and Gibbs Free Energy (these concepts are frequently optional in introductory soil science courses). Redox potential (Eh) represents the tendency or ease with which electrons transfer in a given chemical environment. It is measured with a standard hydrogen electrode. Then, Gibb’s Free Energy (ΔG) is essentially the amount of energy that a reaction yields. The two are related to each other through the Nernst equation:
ΔG0=−nFEh0
Though the details are beyond the scope of this book, you can see from the equation that Gibb’s Free Energy (ΔG) is directly proportional to redox potential (Eh). Going down the redox ladder, Eh decreases, and therefore ΔG also decreases.
This table shows how Eh decreases going down the redox ladder, in volts:
| Reduction Half-Reaction | Eh0 (V) At pH = 0 |
Eh7 (V) At pH = 7 |
|
O2 + 4H+ + 4e– ↔ 2H2O |
1.23 |
0.8 |
|
NO3– + 6H+ + 5e– ↔ 0.5N2 + 3H2O |
1.26 |
0.7 |
|
MnO2 + 2e– + 4H+ ↔ Mn2+ + 2H2O |
0.62* |
0.52* |
|
Fe3+ + e– ↔ Fe2+ |
0.77* |
-0.21 to 0.77* |
|
SO42- + 10H+ + 8e– ↔ H2S + 4H2O |
0.31 |
-0.21 |
|
CO2 + 8H+ + 8e– ↔ CH4 + 4H2O |
0.17 |
-0.24 |
|
2H+ + 2e– ↔ H2 |
0 |
-0.41 |
This figure shows the corresponding amount of energy the reactions in the redox ladder yield:
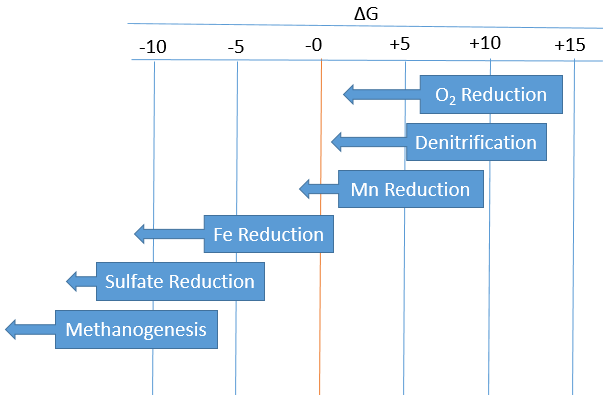
Relative favorability of redox reactions in marine sediments based on energy. Start points of arrows indicate energy associated with half-cell reaction. Lengths of arrows indicate an estimate of Gibb’s free energy (ΔG) for the reaction where a higher ΔG is more energetically favorable (Adapted from Libes, 2011).[3] Note overlap in ΔG ranges; in soil, this results in cases where multiple types of respiration reactions can occur simultaneously.
- Reduction-oxidation features in soil are important indicators
- Gleyed conditions indicate frequent saturation
- Redox depletions and concentrations indicate a fluctuating water table
- The redox ladder shows the order in which microbes use different oxidizing agents, where oxygen (at the top) yields the most energy, and nitrate (2nd from the top) yields the 2nd most energy, and so forth

