22 Wetlands and Other Poorly-Aerated Soils
Rivka Fidel and Amber Anderson
- Explain how flooding and poor aeration influence soil chemical properties and other functions
- Explain the importance of wetlands for humans and wildlife
- Identify where wetlands are likely to be found
- Describe the key characteristics of hydric soils
In previous chapters, we learned that poor drainage and high moisture impede aeration and have a profound impact on soil redox processes. But how does poor aeration affect overall soil functioning? Here we’ll examine the effect of poor aeration on wetland soils and soils that have been temporarily flooded.
General Impacts of Flooding and Poor Aeration
Based on the redox ladder and plants’ need for O2, we can see how flooding and other scenarios resulting in poor aeration would affect soil organisms and holistic soil functioning.
Drowning of typical plant roots
First, as discussed in the soil aeration chapter, typical plants will suffer from insufficient O2 supply to their roots, drowning the roots and eventually causing root rot and plant death. This of course does not apply to plants that naturally grow in poorly aerated soil (see subsection on hydrophytes in the wetlands section below).
Nutrients and toxins
Next, the redox ladder tells us that once O2 gas is depleted, microbes will begin using other, less efficient electron acceptors (oxidizing agents), starting with NO3–, then Mn4+, then Fe3+, and so forth down the ladder. With each step down the ladder, microbes grow more slowly – consequently, they decompose less organic matter, turning less of it into CO2 and allowing more to remain in the soil. For this reason, organic matter tends to accumulate over time in waterlogged, saturated soil. If the flooding is temporary, though, microbes will return to their more rapid aerobic decomposition process and quickly use up the accumulated organic matter.
In the redox ladder, the reduced forms of metals are more soluble. Namely, Mn2+ is more soluble than Mn4+, and Fe2+ is more soluble than Fe3+. Consequently, when these metals get reduced (i.e. accept electrons) following flooding, their solubility increases. Over time, if the floodwater does not drain quickly enough, Mn2+ and Fe2+ may reach toxic concentrations hazardous to typical plants. Beyond metals, certain toxic organic compounds may also accumulate in soil following flooding. Because of the different metabolic pathways involved in anaerobic decomposition (NO3–and further down on the redox ladder), the anaerobic microbes produce different decomposition byproducts that depend both on the electron acceptor and the organic matter being decomposed (the C in the organic matter becomes the electron donor, i.e. the reducing agent). Some of these C-rich decomposition products are toxic to select plants and animals, and may cause stunting or mortality.
The compounds on the bottom of each redox ladder stair step (the reduced forms) are also frequently more mobile in soil, and some are even gases that quickly exit the soil. Namely, when denitrifying bacteria reduce NO3– down to N2 gas, the N2 gas exits the soil. This results in a net loss of soil N, reducing fertility. Similarly, if the soil is flooded for a very long time, SO42- can be reduced down to H2S, resulting in a net loss of soil S. The aforementioned reduced metal forms, Mn2+ and Fe2+, may additionally leach out (albeit slowly) if there is enough drainage following flooding. So some soils may lose cationic metal nutrients following flooding in select circumstances, but the most concerning and impactful nutrient loss in most temporarily flooded agricultural soils is N.
Air and water quality
Gases lost during flooding can furthermore impact air quality and the climate. Although N2 is inert, the denitrification process actually has several steps, and if the process doesn’t proceed all of the way to the end (i.e. if NO3– reduction is partial instead of incomplete), it can release various nitrogen oxides. Here are the denitrification reaction steps with notes regarding their impacts (notice how N is reduced in each half reaction):
- Nitrate reduction: NO3– +2e– → NO2– + O2-
- Nitrate (NO3–) is plant-available, but nitrite (NO2–) is not. So, nitrate reduction lowers plant-available N.
- Nitrite reduction: NO2– + e– → NO + O2-
- Nitric oxide (NO) in the products contributes to acid rain
- Nitric oxide reduction: 2NO + 2e– → N2O + O2-
- Nitrous oxide (N2O), found in the products, is a powerful greenhouse gas
- Nitrous oxide reduction: N2O + e– → N2 + O2-
- This reaction is beneficial as it uses up the greenhouse gas N2O
Beyond the air pollution, any metals or toxins that become soluble during flooding could potentially leach out into groundwater, but because intense flooding requires slow drainage, this process is expected to be inherently slow.


Wetlands and their Soils
Wetlands are a prime example of areas where reducing conditions dominate, and microorganisms from the lower levels of the redox ladder flourish. Here we define wetlands as ecosystems where frequent flooding is both a defining and sustaining feature, such that water has both shaped the ecosystem and become integral to its continued functioning. These ecosystems are home to unique, frequently endangered organisms, and are breeding grounds for many more. Although the means for identifying wetlands vary widely in both science and law, they tend to have 3 key features in common:
- Frequent, regular flooding and saturation (“wetland hydrology”)
- Hydric soils
- Hydrophytic vegetation
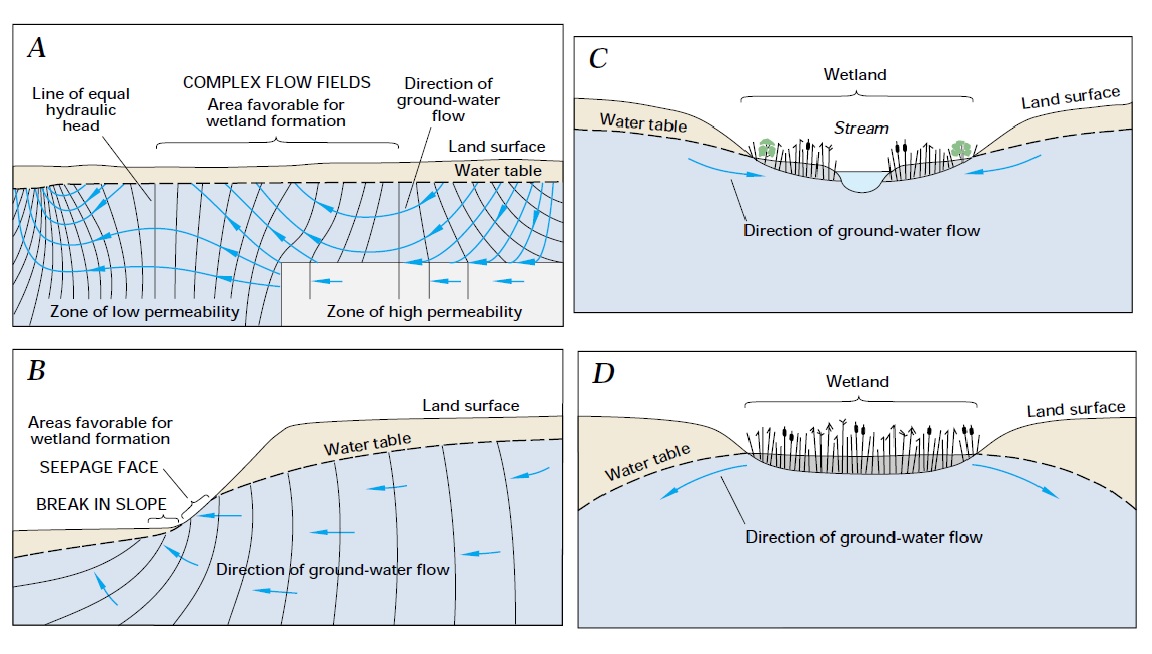
Wetland hydrology
Wetlands, as you might expect, feature very poor drainage. They’re found at low points of landscapes, in closed basins, and areas adjacent to water bodies such as ponds, streams, estuaries, or lagoons. As mentioned in the soil aeration chapter, areas that are low on the landscape have higher clay content, receive water from uphill, and are closer to the water table – so many such areas become inland wetlands like prairie potholes and swamps. Lakes and streams that frequently flood their banks also help to create wetlands, as do coastal zones subject to rising and falling tides. Layers of frozen water, as in permafrost, do not count as wetland hydrology on their own; there must additionally be liquid water present for much of the year.
In some parts of the upper Midwest, such as Central Iowa, closed depressions as common. These soils can be ponded for significant periods during the year, making annual row crops a poor match for the area. Soil features can include dark soil colors to considerable depth, finer soil textures than the surrounding area due to more recent deposition.
This example (below) was dug in the fall, and had collapsed by the spring due to these soil properties. The water seeping into the pit is additionally a sign of poor drainage and wetland hydrology.


Hydric Soils

Over hundreds to thousands of years, soils experiencing wetland hydrology become hydric soils. Hydric soils have the following defining characteristics:
- Gleying (greying) largely due to the reduction of Fe minerals
- Red, orange or yellow colors near plant roots (in contrast with the grey)
- Mottling or other redoximorphic features where patches of gleyed (grey) soil known as redox depletions are interspersed with colored iron concretions (red, orange, yellow, or brown)
- Dark organic matter accumulations due to slow organic matter decomposition under low oxygen conditions
Not all of these soils show all of the properties (at least, not visibly). Many do not show the grey or gleyed colors, as they may have organic matter to significant depth. In those cases, dark colored soils should be examined for red or rusty spots in the dark background to indicate significant wetness. This example (hand pic) is from the landscape above, demonstrates these spots.
Hydric soils fall under multiple soil categories in the USDA soil classification system. Many are Histosols, and others are categorized as having peraquic or aquic moisture regimes. Some include “Aqu” as a suborder root, such as Aquents, Aquepts, and Aqualfs (see the Soil Classification chapter).
The following table shows how much area different types of wetlands take up. Note how wetlands with organic soils (Histosols) compose only 7.3% of all wetlands:
| Data from Eswaren et al (1996), appearing also in The Nature and Properties of Soils by Brady and Weil (2017) | |||
| Wetland type | Global area (1000s km2) | % of ice-free land area | % of all wetland type |
|---|---|---|---|
| Inland (swamps, bogs etc) | 5415 | 3.9 | 28.8 |
| Riparian or ephemeral/seasonal | 3102 | 2.3 | 16.5 |
| Organic (i.e. Histosols) | 1366 | 1.0 | 7.3 |
| Salt-affected, including coastal | 2230 | 1.6 | 11.9 |
| Permafrost-affected (Histels) | 6697 | 4.9 | 35.6 |
Hydrophytic Vegetation
If saturated conditions usually drown plant roots, how do wetland plants survive? In short, they all find some way to get oxygen to the roots. Such water-loving plants are called hydrophytes, from hydro– meaning “water” and –phyte meaning “plant,” “love” and “grow.” Some hydrophytes, like mangroves and cypress trees, keep portions of their roots above the soil; these aboveground root structures, called pneumatophores, then carry oxygen gas to the rest of the roots. Others, like cattails and lotuses, use porous, partly hollow stems called aerenchyma to carry oxygen down to the roots. In both cases, the zone near the roots becomes a source of oxygen instead of a sink; this allows soil microbes next to hydrophyte plant roots to get oxygen and oxidize Fe2+ to Fe3+, resulting in the formation Fe3+ minerals of various colors from red to orange and yellow.




Importance of Wetlands
Wetlands are uniquely integral to whole-Earth functioning due to the processes and functions that their soils support. First, they host unique, exceptionally diverse flora and fauna. Indeed, although wetlands cover only 6% of land on Earth, 40% of plant and animal species live in wetlands either year-round or during breeding seasons. Wetlands furthermore improve water quality in the region by capturing sediment or phosphorus and denitrifying nitrates in the water. Indeed, wetlands are for this reason frequently called the “kidneys of the Earth.” The organic matter accumulations in hydric soils furthermore make them the most carbon-rich soils in the world, and protecting them is therefore integral to fighting climate change.


Where in the world are wetlands?
We’ve learned that wetlands are in frequently flooded areas like closed basins and near water bodies. But where are those located exactly? Below are two databases you can use to find wetlands.
- You can view officially recognized wetlands in the US with the Wetlands Mapper
- The wetlands are hard to see at first, but become clearer as you zoom in
- Globally, protected wetlands are inventoried at Ramsar.org. There are 2 ways to navigate:
- Ramsar Site Map – An interactive map
- Ramsar Site List – Click on a country, then click “view an annotated summary of the sites” to get a pdf with 1 paragraph description per wetland
Draining of Wetlands

As beautiful as wetlands are, most crops do not tolerate saturated conditions, and building infrastructure is very difficult in frequently flooded areas. So, historically, farmers and construction companies have resorted to draining wetlands in order to make a living from the land. The most common soil drainage technique is tile drainage, where perforated pipes are installed underground to help move water down and out of the soil faster. In the US hundreds of thousands of hectares of wetlands have been drained or otherwise destroyed in the past few centuries, and less than half of our original wetland area remains.
Laws Protecting Wetlands
Thankfully, many governments have in recent decades realized the importance of protecting wetlands. In the US, legal implications can arise from draining these areas for agricultural purposes, as outlined in the Wetland Conservation provision of the 1985 Farm Bill, also known as the Swampbuster Act.[1] These lands could be placed in wetland reserve easements with your local NRCS as part of the Wetlands Reserve Program to preserve the wetland functions while receiving financial incentives.[2]

Can Wetlands be Restored?
In a sense, yes, but not completely. Once a wetland has been drained or otherwise severely disturbed, it enters a new system state incapable of supporting its former level of biodiversity and ecosystem services. If drained, O2 enters the soil, and aerobic microbes rapidly decompose the soil organic matter, largely turning it into CO2. What took thousands of years of development can be lost in just a handful of years. While you can re-flood the wetland and re-plant any flora that haven’t gone extinct, allowing most ecosystem services to be largely restored within a few decades, it will take millennia for the soil organic matter to return to what it once was. Furthermore, ecosystem development depends in part on what species were present when at the beginning – and it is impossible to mimic the exact historical conditions with both minimal records and a currently shifting climate. So, while it’s possible to restore most ecosystem services, restored wetlands may never be the same. Similarly, attempts to mimic natural wetlands by constructing man-made ones frequently fall short largely because they haven’t had enough time to develop.
- Poorly aerated soils in general are not suitable for growing typical plants, and frequently have:
- Organic matter accumulations
- Toxic concentrations of transition metal cations like iron (Fe2+) and manganese (Mn2+)
- Toxic organic compounds (usually trace amounts)
- Gaseous losses of N and S, including greenhouse gases and acid rain precursors
- Wetlands are important because, compared to other ecosystems, they are especially good at:
- Filtering water
- Supporting biodiversity
- Storing organic carbon
- Wetlands generally have 3 defining features:
- Wetland hydrology: found in closed basins, depressions, and near water bodies such as lakes, streams, or oceans
- Hydric soils: soils with organic matter accumulations, redoximorphic features (concretions, mottling), and/or gleying
- Hydrophytes: plants that adapted to growing in flooded soil
- The Wetland Conservation provision of the 1985 Farm Bill, also known as the Swampbuster Act, protects wetlands in the US
- After a wetland has been destroyed, it may never be fully restored.
- Most ecosystem services may be restored in years to decades
- The exact species distribution and soil properties are extremely difficult to mimic, especially when records are limited
- Wetlands and other ecosystems take hundreds to thousands of years to fully develop, so we cannot expect them to redevelop fully within our lifetimes, if at all
- Environmental Protection Agency. (2024). CWA Section 404 and Swampbuster: Wetlands on Agricultural Lands. https://www.epa.gov/cwa-404/cwa-section-404-and-swampbuster-wetlands-agricultural-lands ↵
- Natural Resources Conservation Service. (n.d.). Wetlands. https://www.nrcs.usda.gov/conservation-basics/natural-resource-concerns/land/wetlands ↵

