6.0 Fish Nutrition
6.1 Protein
Protein is a notable macronutrient composed or built from subunits called amino acids (AA). Digestive actions break apart the dietary proteins to make AA available for absorption into the blood. Hence, the nutritional term digestible protein. The animal can metabolically modify some AA to other AA. Some AAs cannot be metabolically created in the animal from other AAs available from the diet. The analyzed protein levels are listed on the analysis tag of the commercial feed. Typically, the AA content is not determined and listed for each batch of feed manufacturing.
Amino acids
Amino acids must also be provided or available in the diet in the correct balance. They must be in a digestible form and available from the ingested diet. With the proper availability of AA, proteins can be built for the needed protein structures in a living animal. The same 20 AA are sought in many animal diets. AA that cannot be formed from other dietary AA is essential in the diet.
Indispensable AA is known as AA which must be obtained from the diet. Indispensable AA deficiency will result in numerous disease manifestations, reduced performance, and weight gain. The list of Indispensable AA is shown in Table 1. Fish are generally similar to other animals, although AA requirements may vary or be specific to a species, life stage, gender, and age/health. As a rule of thumb, consider the 10 AA listed as universal unless described differently for a specific species or publication. Ten amino acids are Indispensable l AA for animals, including fish. The list of Indispensable AA can be remembered by the mnemonic PVT TIM HALL. Table 1 below lists the AA with the letters of the mnemonic:
|
P |
Phenylalanine |
|
V |
Valine |
|
T |
Threonine |
|
T |
Tryptophan |
|
I |
Isoleucine |
|
M |
Methionine |
|
H |
Histidine |
|
A |
Arginine |
|
L |
Lysine |
|
L |
Leucine |
While the same indispensable amino acids are listed across species, there can be differences between species in the ratios of the combination, making up the total amino acid requirements. In 1986 Wilson reported that Chinook salmon require 6.0% of the total protein to be arginine, while Rainbow trout needs 4.0%. Both fish are salmonids and can inhabit similar biotopes.[1].
It may be relatively safe to assume all living organisms utilize the same 20 amino acids in protein construction along with the occasional use of selenocysteine [2] and pyrrolysine.[3] Significant differences exist in the metabolic pathways and sources of whatever essential AA is required for a specific species. Aquatic invertebrates may muster additional metabolic resources from metabolic pathways and even the gut microbiome. [4]. While the same essential amino acids are listed across species, there can be differences between species in the ratios of the combination, making up the total amino acid requirements.
As mentioned in Chapter 3 regarding food intake by mouth position, aquatic animals in captivity may need more understanding of the nutritional requirements. Currently, some aquatic livestock industry segments provide “trash fish” to their captive fish because formulated feeds are not yet available. Trash fish, for the newcomer readers, is a byproduct of fisheries. Trash fish is the by-catch after food fish are separated from the seining. Indispensable amino acids can be one of the big hurdles to overcome in finding a diet to support the health and life cycle of the captive species. Still, the aquatic livestock industry depends on fish-meal for protein and oil sources. The fish meal protein is high-quality and digestible, with balanced AA ratios for many cultured species. Trouble is coming for the aquatic livestock industries and the fish-meal suppliers to keep pace with the market demand as both aquatic and terrestrial livestock utilize fish meal. The world fisheries for fish-meal production are reaching the limit of sustainability. The aquatic demand side is growing faster than the terrestrial side is shrinking. There are carnivorous, omnivorous, and herbivorous species of fish.
Typically the youngest life stages require the highest dietary protein levels, which taper off with maturity. Juvenile fish require higher protein levels that may need 30-50 % [5]. The fry of all species can be regarded as carnivorous when cannibalism sets into the population.[6]. Hatcheries depend on fish-meal and are fortunate that the quantity is relatively small.
Crude protein
Crude protein (CP)is part of the typical chemical analysis that feeds are evaluated for. The nutrition tag on bagged feed has a list of ingredients that may have many protein sources. Crude protein does not describe digestible protein. Feathers, for example, do not have much digestible protein because the animal gut cannot break some of the proteins apart (digestion) to free the AA for absorption. If AAs are not free to be absorbed through the gut wall, they cannot be metabolized for constructing the specific proteins needed at the time.
While the same Indispensable amino acids are listed across species, there can be differences between species in the ratios of the combination, making up the total amino acid requirements.
Digestible protein
Digestible protein (DP) is the portion of the CP fed absorbed from the small intestine. This protein is also called absorbed protein (AP) and is not calculated from a laboratory chemical assay. Nutritional studies done in live animals can establish the amount of AP taken out of the ingested crude protein (IP) by subtracting the excreted fecal protein (FP) from the IP protein ingested. Fecal content for fish nutritional studies is typically collected by sedimentation in tank water or fecal stripping by manually expressing feces from the rectum. The sedimentation method is typically lower-valued than stripping due to the leaching of many nutrients while in water.[7]
IP – FP = AP
The digestible protein then gets the analysis closer to the metabolized protein (MP) available to metabolize. On the side of caution, do not assume that all protein sources of the same name are equal in MP. The MP value of a protein source lot or batch could be altered with various abuse such as heat of storage, processing, time since harvest, source strain, or species of the ingredient for the product. While the product manufacture and content are variables in protein availability, so are the conditions the fish are cultured in. A feed will produce different outcomes when crucial factors impacting metabolism vary. Crucial factors can be temperature, feeding strategy, and flow rate. For example, two production seasons may have different efficiency due to weather or other factors beyond the control of some operations. These are the more technical issues that the experienced livestock producer could benefit from evaluating.
Biological Value
Biological Value (BV) indicates how suitable the AA of the MP source matches the metabolic needs of the animal. It measures protein quality by calculating the nitrogen ratio used for protein synthesis or tissue formation and the nitrogen absorbed from the feed. There are many sources of protein to put in feeds. The CP can be chemically determined. The nutritional study will yield the MP. A value is still needed to determine how appropriate a feed may be for the animal eating it. Determining the BV of protein sources may indicate that a diet for different life stages to be cost-effective.
| Feed | BV |
| Concentrated whey | 104 |
| Egg | 100 |
| Milk (cow) | 91 |
| Beef (lean) | 80 |
| Casein from whey | 77 |
| Soybean meal | 74 |
| Wheat gluten | 64 |
| Corn zein | 35 |
When evaluating protein sources, the value of the feed rests in the BV or metabolic utilization of the protein source to the animal eating the protein. Table 2 is the information from studying how appropriate the listed protein source is for monogastrics. The source is likely terrestrial monogastrics. Nonetheless, the list shows the distinct variation amongst commonly used protein sources. The feedstuffs with the highest BV typically come with a higher unit cost. It is safe to assume that higher quality (>BV) feeds come at a higher price. Depending on the discussion of specific fish species, the list of protein sources may be digestible, metabolizable, and biologically utilized. BV is a necessary component in the calculations around the economy of a protein source. Understanding the nutritional requirements of a fish species is paramount to successfully determining the most cost-effective protein source. Nutritional requirements are being defined for species that are called economically important species, such as catfish (Siluriformes), trout and salmon (Salmonids), and a few others.[8] [9].
With the previous information, we understand that a high-quality source of protein is sought that will yield the greatest results in the livestock. Digestion of dietary proteins will create the individual AA that will be absorbed and metabolized. It is appropriate to ask ourselves about the body’s uses of this AA influx from the intestines. Proteins are integral to a maintenance cycle in muscle, for example. As previously used proteins are replaced with new proteins, the old protein is broken down and becomes AA, and some portion is reused in new protein generation. Enzymes do this salvage process. The non-reuse of former protein AA or the need for different AA in the protein construct causes the need for replacement AA from the diet.
Muscle is likely the largest protein user, especially in the young growing animal. While protein is necessary for most tissues, the increasing muscle mass in the young animal produces the meat utilized in the human diet. It is simple to say and understand that growth is a situation where the synthesis of new protein is greater than the destruction of old protein. This process is termed protein accretion. Generally, this means a dietary supply of MP with an AA profile similar to the animal’s muscle. Because growth is limited and declines with age and maturity, the amount of MP required per kg of weight gain declines. The energy is increasingly shunted to energy stores of fat and leads to declining muscle mass (protein).
Figure 6.1 depicts the AA assays from several species and the organ meats. The AA composition is similar but differs most from AA lysine. The great diversity of fishes is one area that requires some thought. It was stated previously that Chinook salmon require 6% arginine of the total protein while Rainbow trout require 4% of the same AA. The adjoining table[10] shows the AA analysis of muscle tissue and organs from several livestock species.
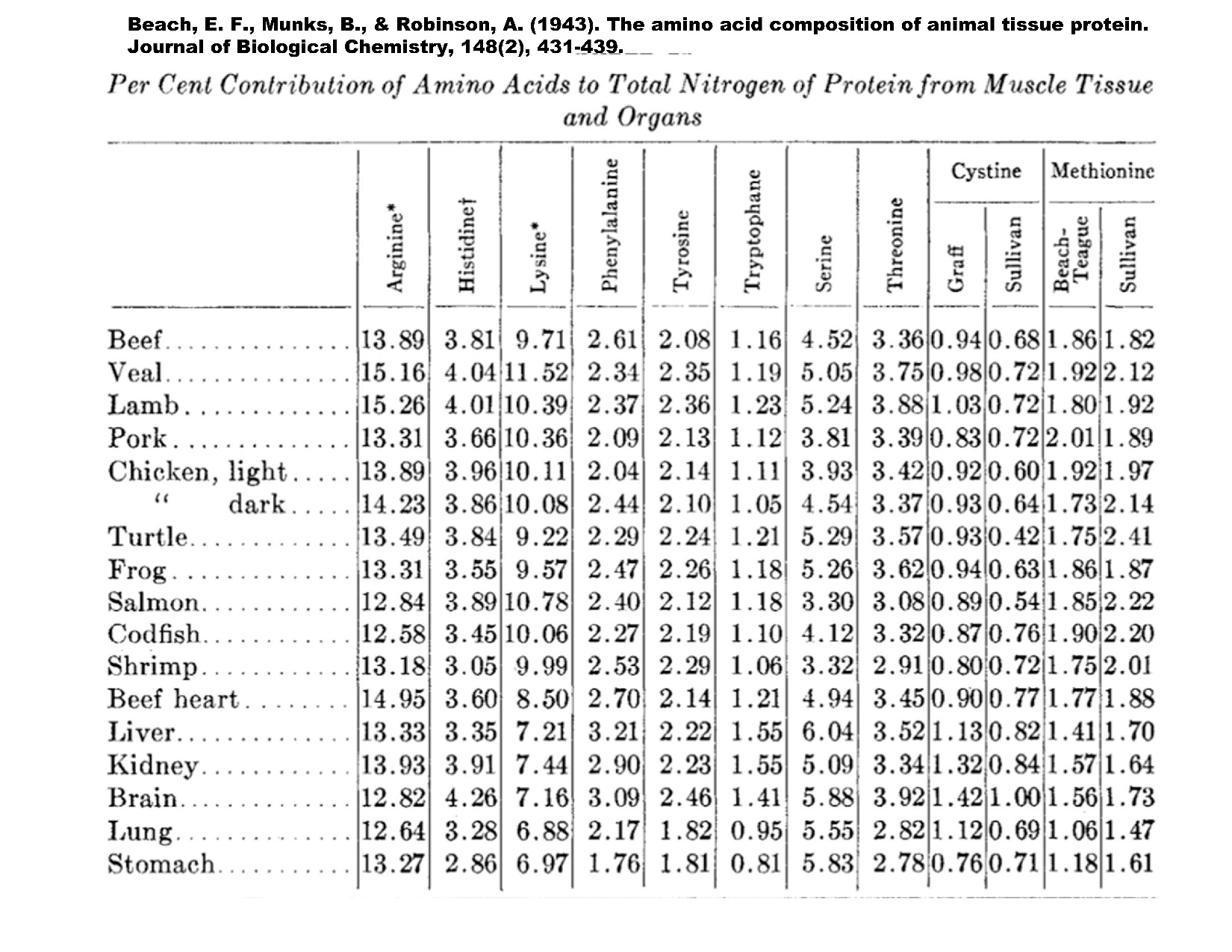
After growth through muscle creation, the reproduction process also has large dietary protein requirements. Given that reproduction is a high-priority function for species survival. The MP AA profile should match that required for gamete production. This requirement can be considerable in some fish species. For example, Salmon eggs could make up almost 30% of the body mass. The exaggerated gamete to body mass is compounded when many species quit eating at or around the time of spawning. The process of spawning for many fish is extremely stressful. The reliance on nutrient stores is significant. These nutrient stores mean the catabolic status takes over the metabolism. In the case of anadromous fish, such as various species of Pacific salmon (Oncorhynchus spp.), this catabolism is so severe, so complete the broodstock dies after reaching the waters of origin and completing the task of spawning. The task of migrating from the ocean to the home spawning is complete without nutrition, as the energy needed brings about catabolism. Catabolism is the withdrawal of protein from the body structures to meet the migration energy demands of the long journey to their waters of origin. [11].
Recalling the previous statement that newly hatched and small fry will do better on high protein diets closely formulated to their muscle AA analysis, we can turn our attention to the optimal growth diet of grow-out fish. These fish have gained in size and moved to larger feed. This life stage is varied depending on the species being reared. Optimal growth for many cultured species is around 32-35% protein of good quality. Protein is also an energy source for animals. Fish are good at using protein wherever needed to fulfill the body’s needs for tissue or energy.
- Wilson, R. P. (1986). Protein and amino acid requirements of fishes. Annual review of nutrition, 6, 225-244 ↵
- Böck, A. (2000). biosynthesis of selenoproteins–an overview. Biofactors, 11(1-2), 77-78 ↵
- Srinivasan, G., James, C. M., & Krzycki, J. A. (2002). Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA. Science, 296(5572), 1459-1462 ↵
- Armitage, M. E., Raymont, J. E. G., & Morris, R. J. (1981). Amino acid synthesis in Neomysis integer and Gnathophausia sp. Feeding experiments using 14C-labelled precursors. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 68(2), 183-191 ↵
- Jobling, M. (2012). National Research Council (NRC): Nutrient requirements of fish and shrimp ↵
- Polis, G. A. (1981). The evolution and dynamics of intraspecific predation. Annual Review of Ecology and Systematics, 12, 225-251 ↵
- Fernández, F., Miquel, A. G., Cumplido, L. R., Guinea, J., & Ros, E. (1996). Comparisons of fecal collection methods for digestibility determinations in gilthead sea bream. Journal of fish biology, 49(4), 735-738 ↵
- Jobling, M. (2012). National Research Council (NRC): Nutrient requirements of fish and shrimp ↵
- Wilson, R. P., & Moreau, Y. (1996). Nutrient requirements of catfishes (Siluroidei). Aquatic Living Resources, 9, 103-111 ↵
- Beach, E. F., Munks, B., & Robinson, A. (1943). The amino acid composition of animal tissue protein. Journal of Biological Chemistry, 148(2), 431-439 ↵
- Miller, K. M., Schulze, A. D., Ginther, N., Li, S., Patterson, D. A., Farrell, A. P., & Hinch, S. G. (2009) Salmon spawning migration: metabolic shifts and environmental triggers. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 4(2), 75-89 ↵
- Bilby, R. E., Beach, E. W., Fransen, B. R., Walter, J. K., & Bisson, P. A. (2003). Transfer of nutrients from spawning salmon to riparian vegetation in western Washington. Transactions of the American Fisheries Society, 132(4), 733-745 ↵

