2.2 Osmosis and Enzymatic Browning
Objectives
- Observe osmosis.
- Understand how osmosis can be used to move water in and out of raw fruits and vegetables.
- Observe enzymatic browning.
- Identify some methods of controlling enzymatic browning.
Osmosis
Definition: Water moves across a semipermeable membrane in response to solute concentration. Osmosis is important when considering fruit and vegetable cell membranes.
Cell membranes are semipermeable, which:
- Allows water to pass in and out of cells
- Blocks passage of dissolved solutes
This means water will move across the cell membrane to try to even out concentrations. Cells full of water = crisp fruits and vegetables.
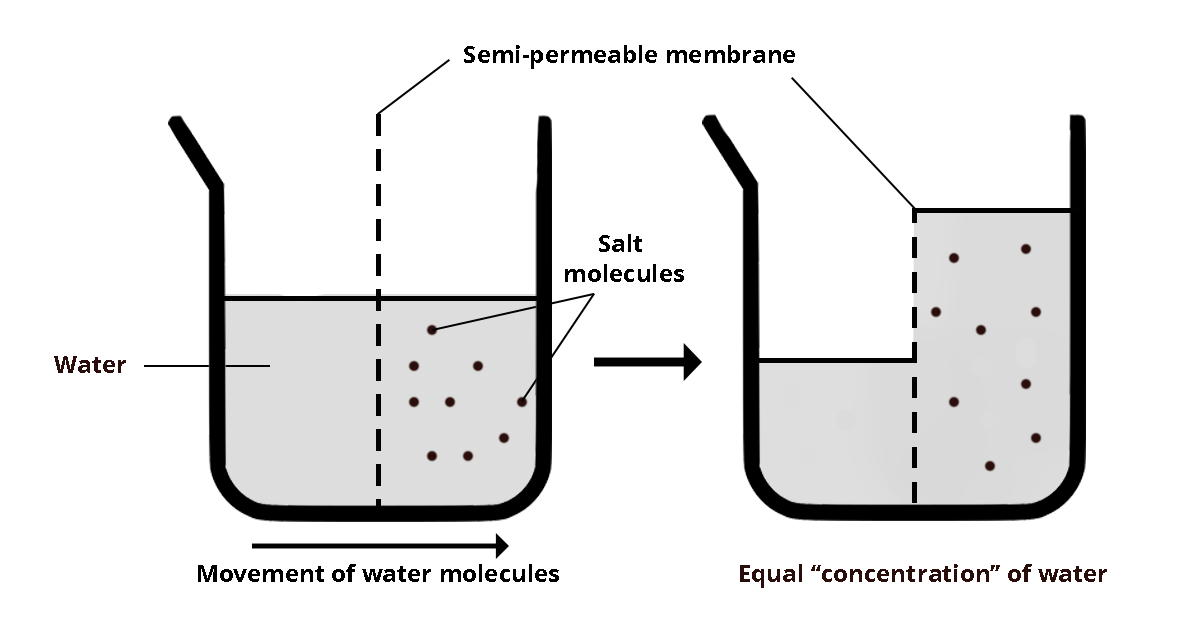
Importance in food quality: Osmosis may lead to a soggy, not crisp texture in fruits and vegetables
Give an example of osmosis in a food product:
Observe the effect of salt on the appearance and texture of cucumbers
| Method | Appearance | Texture |
|---|---|---|
| Soak in 1 cup of cold water only | ||
| Soak in salt/water solution (2 tbsp salt to 1 cup cold water) | ||
| Sprinkle with 2 tbsp salt only |
Explanation:
Enzymatic Browning
Definition: Browning reaction when some fruits and vegetables are cut and exposed to oxygen. Substrate + Enzyme + Oxygen = Browning –OR– Phenolic compounds + Polyphenoloxidase + Oxygen
Importance in food quality: Appearance (not a safety concern)

Give an example of enzymatic browning in a food product:
Give an example of a way to prevent enzymatic browning:
Demonstrate enzymatic browning and methods of control
- Slice an apple or banana onto separate dishes with a stainless steel knife.
- Apply assigned treatment.
- Allow to stand uncovered for one hour.
- Record observations.
| Treatment | Appearance |
|---|---|
| None | |
| Diluted lemon juice (1 part to 3 parts water) |
|
| Commercial anti-darkening agent |

