6 Membranes
Introduction to Membrane Processes and Modeling Porous Membranes
![]() = pressure driving force across the membrane (pressure)
= pressure driving force across the membrane (pressure)
![]() = membrane thickness (length)
= membrane thickness (length)
![]() = membrane porosity; volume of pores per unit volume of membrane (unitless)
= membrane porosity; volume of pores per unit volume of membrane (unitless)
![]() = permeate viscosity (cP) or (mass length-1 time-1)
= permeate viscosity (cP) or (mass length-1 time-1)
![]() = fluid density (mass volume-1)
= fluid density (mass volume-1)
![]() = membrane tortuosity factor (>1)
= membrane tortuosity factor (>1)
![]() = membrane cross-sectional area (area)
= membrane cross-sectional area (area)
![]() = total pore surface area per volume of membrane solid material (area volume-1)
= total pore surface area per volume of membrane solid material (area volume-1)
![]() = pore diameter (length)
= pore diameter (length)
![]() = hydraulic pore diameter (length)
= hydraulic pore diameter (length)
![]() = permeate flux (vol area-1 time-1) or (length time-1)
= permeate flux (vol area-1 time-1) or (length time-1)
![]() = permeability of component i (length2)
= permeability of component i (length2)
![]() = pore length
= pore length
![]() = membrane thickness (length)
= membrane thickness (length)
![]() = number of pores per unit of flow area (i.e. top down, not a cross-section) of membrane
= number of pores per unit of flow area (i.e. top down, not a cross-section) of membrane
![]() = molar flux through the membrane per unit area (mol time-1 area-1)
= molar flux through the membrane per unit area (mol time-1 area-1)
![]() = Reynold’s number (unitless)
= Reynold’s number (unitless)
![]() = pressure (pressure)
= pressure (pressure)
![]() = pressure at the surface of the pore (pressure)
= pressure at the surface of the pore (pressure)
![]() = pressure at position L within the membrane pore (pressure)
= pressure at position L within the membrane pore (pressure)
![]() = permeability of the membrane to species i (length2 time-1)
= permeability of the membrane to species i (length2 time-1)
![]() = permeance of the membrane to species i (length time-1)
= permeance of the membrane to species i (length time-1)
![]() = resistance of the filter cake (length-1)
= resistance of the filter cake (length-1)
![]() = resistance of component i (length-1)
= resistance of component i (length-1)
![]() = resistance of the membrane (length-1)
= resistance of the membrane (length-1)
![]() = time (time)
= time (time)
![]() = superficial velocity of the permeate (length time-1)
= superficial velocity of the permeate (length time-1)
![]() = flow velocity (length time-1)
= flow velocity (length time-1)
![]() = cumulative volume of permeate collected since the start of the filtration (volume)
= cumulative volume of permeate collected since the start of the filtration (volume)
![]() = direction of flux (length)
= direction of flux (length)
General Flux Equation
(25.1) ![]()
Dead-end Filtration
(25.2) ![]()
(25.3) ![]()
(25.4) ![]()
Modeling of Porous Membranes
Hagen-Poiseuille Law
(25.5) ![]()
*eq 25.5 is restricted to ![]() , where
, where ![]()
(25.6) ![]()
Ideal porous membrane: straight pores of uniform diameter
(25.7) ![]()
Compensation for tortuous pores and variation in pore diameter
(25.8) ![]()
(25.9) ![]()
(25.10) ![]()
Example
A membrane of thickness 0.003 cm will be used to filter room-temperature water. In order to justify the cost of the membrane, we need to filter 200 m3 of water every day per m2 of membrane purchased. We are able to maintain a pressure of 50 kPa on the permeate side. What pressure do we need to apply on the retentate side? Ignore any resistance from the retentate. Assume operation with an ideal porous membrane with a porosity of 35% and pore diameter of 0.2 μm.
Porous Membranes
![]() = internal parameter (length mass-1)
= internal parameter (length mass-1)
![]() = pressure driving force across the membrane (pressure)
= pressure driving force across the membrane (pressure)
![]() = pressure across the membrane during the constant pressure segment of combined operation (pressure)
= pressure across the membrane during the constant pressure segment of combined operation (pressure)
![]() = filter cake porosity; volume of void space per unit volume of filter cake (unitless)
= filter cake porosity; volume of void space per unit volume of filter cake (unitless)
![]() = permeate viscosity (cP) or (mass length-1 time-1)
= permeate viscosity (cP) or (mass length-1 time-1)
![]() = filter cake density (mass volume-1)
= filter cake density (mass volume-1)
![]() = surface area of the accumulated filter cake (area)
= surface area of the accumulated filter cake (area)
![]() = surface area of the membrane (area)
= surface area of the membrane (area)
![]() = concentration of solid material per unit volume of feed (mass volume-1)
= concentration of solid material per unit volume of feed (mass volume-1)
![]() = effective diameter of cake particles (length)
= effective diameter of cake particles (length)
![]() = permeate flux (volume area-1 time-1) or (length time-1)
= permeate flux (volume area-1 time-1) or (length time-1)
![]() = internal parameter used in modeling constant pressure operation (volume2 hr-1)
= internal parameter used in modeling constant pressure operation (volume2 hr-1)
![]() = internal parameter, function of effective particle diameter (length-2)
= internal parameter, function of effective particle diameter (length-2)
![]() = parameter used in modeling combined constant flux/constant pressure operation, equals
= parameter used in modeling combined constant flux/constant pressure operation, equals ![]() (length mass-1)
(length mass-1)
![]() = thickness of accumulated filter cake (length)
= thickness of accumulated filter cake (length)
![]() = resistance of the accumulating filter cake (length-1)
= resistance of the accumulating filter cake (length-1)
![]() = resistance of the membrane (length-1)
= resistance of the membrane (length-1)
![]() = time (time)
= time (time)
![]() = total time elapsed during the constant flux operation mode (time)
= total time elapsed during the constant flux operation mode (time)
![]() = target permeate flux value (volume time-1)
= target permeate flux value (volume time-1)
![]() = cumulative volume of permeate collected since the start of the filtration (volume)
= cumulative volume of permeate collected since the start of the filtration (volume)
![]() = internal parameter for modeling constant pressure operation (volume)
= internal parameter for modeling constant pressure operation (volume)
![]() = total permeate collected during the constant flux operation mode (volume)
= total permeate collected during the constant flux operation mode (volume)
Resistance from Filter Cake
(26.1) ![]()
(26.2) ![]()
for large, relatively flat membranes
(26.3) ![]()
An equation for the cake resistance, ![]() , for capillary or hollow fiber membranes given in Seader (14-22).
, for capillary or hollow fiber membranes given in Seader (14-22).
Operation with Constant Pressure (Flux Decreases with Time)
(26.4) ![]()
(26.5) ![]()
(26.6) ![]()
(26.7) ![]()
(26.8) ![]()
Operation with Constant Flux (Applied Pressure Drop Increases with Time)
(26.9) ![]()
Combined Operation: Constant Flux to Maximum Pressure Drop, Then Continue at Constant Pressure with Decreasing Flux
(26.10) ![Rendered by QuickLaTeX.com \begin{eqnarray*} V(t)=\frac{-R_mA_M}{K_2c_F} + [\left(\frac{A_MR_m}{K_2c_F}\right)^2\\+\frac{2A_M}{K_2c_F}\left(R_mV_{CF}+\frac{0.5K_2c_FV_{CF}^2}{A_M}+\frac{A_M\Delta P_{UL}\left(t-t_{CF}\right)}{\mu}\right) ]^{0.5} \end{eqnarray*}](https://iastate.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-e70b9d0946cf474be29d42f66aa20c11_l3.png)
(26.11) ![Rendered by QuickLaTeX.com \begin{eqnarray*} J(t)=\frac{A_M\Delta P_{UL}}{K_2c_F\mu} [\left(\frac{A_MR_M}{K_2c_F}\right)^2\\+\frac{2A_M}{K_2c_F}\left(R_mV_{CF}+\frac{0.5K_2c_FV_{CF}^2}{A_M}+\frac{A_M\Delta P_{UL}\left(t-t_{CF}\right)}{\mu}\right) ]^{-0.5} \end{eqnarray*}](https://iastate.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-47947496c2bf4dad2faf2e2691aad812_l3.png)
(26.12) ![]()
Example
We aim to use a flat, porous membrane to filter milk. The membrane has a surface area of 17.3 cm2; the membrane resistance and thickness are not known. The milk contains 4.3 kg/m3 solids and has a viscosity of 0.001 Pa-s. We previously filtered this milk at an applied pressure drop of 20 psi and the following data was collected:
| Time (hr) | 0.5 | 1.0 | 1.5 | 2.0 |
|---|---|---|---|---|
| Total volume collected (L) | 0.31 | 0.40 | 0.53 | 0.61 |
- How much filtered milk could be collected over a 12-hour period if we operate at
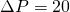 psi?
psi? - How much filtered milk could be collected over a 12-hour period if we operate at
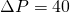 psi?
psi? - If we operate in constant flux mode at 0.1 L/hr, how long will it take to reach our maximum allowable
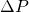 of 40 psi? How much permeate would be collected during this time?
of 40 psi? How much permeate would be collected during this time? - If we operate in combined mode for 24 hours with a constant flux of 0.1 L/hr and maximum allowable pressure drop of 40 psi, how much permeate would be collected?
Nonporous Membranes Gas Permeation
![]() = ideal separation factor of species A and B (unitless)
= ideal separation factor of species A and B (unitless)
![]() = actual separation factor of species A and B (unitless)
= actual separation factor of species A and B (unitless)
![]() = diffusivity of species i in the membrane (length2 time-1)
= diffusivity of species i in the membrane (length2 time-1)
![]() = Henry’s Law coefficient of species i in the membrane (mol volume-1 pressure-1)
= Henry’s Law coefficient of species i in the membrane (mol volume-1 pressure-1)
![]() = membrane thickness (length)
= membrane thickness (length)
![]() = molar transmembrane flux of species i (mol area-1 time-1)
= molar transmembrane flux of species i (mol area-1 time-1)
![]() = total pressure of the feed (pressure)
= total pressure of the feed (pressure)
![]() = partial pressure of species i at the membrane on the feed side (pressure)
= partial pressure of species i at the membrane on the feed side (pressure)
![]() = partial pressure of species i in the bulk feed (pressure)
= partial pressure of species i in the bulk feed (pressure)
![]() = partial pressure of species i at the membrane on the permeate side (pressure)
= partial pressure of species i at the membrane on the permeate side (pressure)
![]() = partial pressure of species i in the bulk permeate (pressure)
= partial pressure of species i in the bulk permeate (pressure)
![]() = permeability of the membrane to species i (length2 time-1)
= permeability of the membrane to species i (length2 time-1)
![]() = permeance of the membrane to species i (length time-1)
= permeance of the membrane to species i (length time-1)
![]() = total pressure of the permeate (pressure)
= total pressure of the permeate (pressure)
![]() = pressure ratio (unitless)
= pressure ratio (unitless)
![]() = mole fraction of species i on the feed side
= mole fraction of species i on the feed side
![]() = mole fraction of species i in the permeate
= mole fraction of species i in the permeate
Gas through a non-porous membrane
(27.1) ![]()
if film resistance is negligible
(27.2) ![]()
(27.3) ![]()
(27.4) ![]()
(27.5) ![]()
(27.6) ![]()
when A and B are the only components of the feed and permeate, so that
(27.7) ![]()
(27.8) ![]()
Example
A certain membrane has an ideal separation factor of 5.12 for O2 (A) and N2 (B). It has been proposed to use this membrane to separate O2 from air. If our feed pressure is 5.0 atm and our permeate pressure is maintained at 0.25 atm, what is the composition of our product gas?
Dialysis
![]() = log mean concentration difference (mol volume-1)
= log mean concentration difference (mol volume-1)
![]() = area of membrane cross-sectional to the flow path (area)
= area of membrane cross-sectional to the flow path (area)
![]() = concentration of species i on the feed side of the membrane (mol volume-1)
= concentration of species i on the feed side of the membrane (mol volume-1)
![]() = concentration of species i on the permeate side of the membrane (mol volume-1)
= concentration of species i on the permeate side of the membrane (mol volume-1)
![]() = concentration of species i in the retentate (mol volume-1)
= concentration of species i in the retentate (mol volume-1)
![]() = concentration of species i in the wash solution (mol volume-1)
= concentration of species i in the wash solution (mol volume-1)
![]() = mass transfer coefficient of species i in the feed (length time-1)
= mass transfer coefficient of species i in the feed (length time-1)
![]() = mass transfer coefficient of species i in the permeate (length time-1)
= mass transfer coefficient of species i in the permeate (length time-1)
![]() = overall mass transfer coefficient of species i (length time-1)
= overall mass transfer coefficient of species i (length time-1)
![]() = thickness of the membrane (length)
= thickness of the membrane (length)
![]() = rate of mass transfer of species i (mol time-1)
= rate of mass transfer of species i (mol time-1)
![]() = permeability of the membrane to species i (length2 time-1)
= permeability of the membrane to species i (length2 time-1)
Transport across a small membrane segment
(28.1) ![]()
(28.2) ![]()
(28.3) ![]()
For counter-current operation:
(28.4) ![Rendered by QuickLaTeX.com \begin{displaymath} ({\Delta}c_i)_{\rm LM} = \frac{(c_{i_F}-c_{i_P})-(c_{i_R}-c_{i,\rm wash})}{\ln\left[{\frac{(c_{i_F}-c_{i_P})}{(c_{i_R}-c_{i,\rm wash})}}}\right] \end{displaymath}](https://iastate.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-0f879bcff20c84e477fded98d5b1eb1f_l3.png)
Water (solvent) transport number = water (solvent) flux / solute flux
Example
We aim to recover 30% of the H2SO4 from a 0.78 m3/hr feed containing 300 kg/m3 of H2SO4 and smaller amounts of CuSO4 and NiSO4. We have up to 1.0 m3/hr of water available as a wash stream. The process is to run counter-current and at 25°C. The available membrane has an H2SO4 permeance of 0.025 cm/min, negligible permeance to the other sulfates, and a water transport number (mass) of +1.5. Previous experience suggests that ![]() 1/(0.020 cm/min). What is the required membrane area and the volumetric flowrate of the two streams exiting the dialysis unit?
1/(0.020 cm/min). What is the required membrane area and the volumetric flowrate of the two streams exiting the dialysis unit?
Reverse Osmosis
![]() = pressure drop across the membrane (pressure)
= pressure drop across the membrane (pressure)
![]() = osmotic pressure drop across the membrane (pressure)
= osmotic pressure drop across the membrane (pressure)
![]() = activity coefficient of the solvent on the feed/retentate side (unitless)
= activity coefficient of the solvent on the feed/retentate side (unitless)
![]() = concentration polarization factor (unitless)
= concentration polarization factor (unitless)
![]() = viscosity of the feed solution (cP)
= viscosity of the feed solution (cP)
![]() = osmotic pressure (pressure)
= osmotic pressure (pressure)
![]() = density of the feed solution (mass volume-1)
= density of the feed solution (mass volume-1)
![]() = internal parameter used in estimating ki (unitless)
= internal parameter used in estimating ki (unitless)
![]() = internal parameter used in estimating ki (unitless)
= internal parameter used in estimating ki (unitless)
![]() = concentration of species i in feed/permeate (mol volume-1)
= concentration of species i in feed/permeate (mol volume-1)
![]() = concentration of solute in the permeate (mol volume-1) or (mass volume-1)
= concentration of solute in the permeate (mol volume-1) or (mass volume-1)
![]() = concentration of solute in the feed (mol volume-1) or (mass volume-1)
= concentration of solute in the feed (mol volume-1) or (mass volume-1)
![]() = internal parameter used in estimating
= internal parameter used in estimating ![]() (unitless)
(unitless)
![]() = tube diameter (length)
= tube diameter (length)
![]() = hydraulic diameter (length)
= hydraulic diameter (length)
![]() = diffusivity of species i in the indicated solvent (length2 time-1)
= diffusivity of species i in the indicated solvent (length2 time-1)
![]() = height of flow channel (length)
= height of flow channel (length)
![]() = mass transfer coefficient of species i (length time-1)
= mass transfer coefficient of species i (length time-1)
![]() = molar flux of solvent A through the membrane (mol area-1 time-1)
= molar flux of solvent A through the membrane (mol area-1 time-1)
![]() = Reynold’s number of the feed/retentate (unitless)
= Reynold’s number of the feed/retentate (unitless)
![]() = Schmidt number of the feed/retentate (unitless)
= Schmidt number of the feed/retentate (unitless)
![]() = universal gas constant (pressure volume mol-1 temperature-1) or (energy mol temperature-1)
= universal gas constant (pressure volume mol-1 temperature-1) or (energy mol temperature-1)
![]() = salt (solute) passage number (unitless)
= salt (solute) passage number (unitless)
![]() = salt (solute) rejection factor (unitless)
= salt (solute) rejection factor (unitless)
![]() = system temperature (temperature)
= system temperature (temperature)
![]() = velocity of the feed (length time-1)
= velocity of the feed (length time-1)
![]() = specific volume of the solvent (volume mol-1)
= specific volume of the solvent (volume mol-1)
![]() = width of flow channel (length)
= width of flow channel (length)
![]() = mole fraction of species i on the feed/retentate side (unitless)
= mole fraction of species i on the feed/retentate side (unitless)
(29.1) ![]()
when ![]() and
and ![]() then
then
(29.2) ![]()
if ![]() is sufficiently small, then
is sufficiently small, then ![]() and
and
(29.3) ![]()
(29.4) ![]()
(29.5) ![]()
(29.6) ![]()
(29.7) ![]()
for a circular tube, ![]()
for a rectangular channel, ![]()
during turbulent flow ![]()
![]()
during laminar flow, circular tube ![]()
![]()
during laminar flow, rectangular channel ![]()
![]()
(29.8) ![]()
(29.9) ![]()
(29.10) ![]()
Watch a video from LearnChemE that explains osmotic pressure: Osmotic Pressure Derivation (5:00).
Example
We intend to use reverse osmosis on a feed stream containing 1.8 wt% NaCl to produce water containing 0.05 wt% NaCl. The separation is to take place at 25°C with a feed side pressure of 1,000 psia and a permeate-side pressure of 50 psia. The proposed membrane has permeance of ![]() g/cm2-s-atm for water.
g/cm2-s-atm for water.
(a) Ignoring resistances to mass transfer, how much water can be produced per day per unit area of membrane?
(b) If ![]() = 0.005 cm/s, what is the concentration polarization factor?
= 0.005 cm/s, what is the concentration polarization factor?
Pervaporation
![]() = activity coefficient of species i (unitless)
= activity coefficient of species i (unitless)
![]() = system-specific parameters for the van Laar model
= system-specific parameters for the van Laar model
![]() = membrane thickness (length)
= membrane thickness (length)
![]() = saturated vapor pressure of species i, function of temperature and Antoine equation coefficients (pressure)
= saturated vapor pressure of species i, function of temperature and Antoine equation coefficients (pressure)
![]() = permeability of the membrane to species i (length2 time-1)
= permeability of the membrane to species i (length2 time-1)
![]() = total pressure on permeate side (pressure)
= total pressure on permeate side (pressure)
![]() = mole fraction of species i on the feed/retentate side (unitless)
= mole fraction of species i on the feed/retentate side (unitless)
![]() = mole fraction of species i on the permeate side (unitless)
= mole fraction of species i on the permeate side (unitless)
(30.1) ![]()
van Laar model for activity coefficients, binary system
(30.2) ![Rendered by QuickLaTeX.com \begin{equation*} \ln\gamma_1=\frac{A_{12}}{\left[1+\frac{x_1A_{12}}{x_2A_{21}}\right]^2} \end{equation*}](https://iastate.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-bc297ba6fa1e00858b8c3881cbf696bc_l3.png)
(30.3) ![Rendered by QuickLaTeX.com \begin{equation*} \ln\gamma_2=\frac{A_{21}}{\left[1+\frac{x_2A_{21}}{x_1A_{12}}\right]^2} \end{equation*}](https://iastate.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-839a0fe3e5217dad2bcf9a6e23b1b4e0_l3.png)
Example
We have obtained a potential pervaporation membrane and we aim to use it to separate ethanol and water. We tested this system at 60°C, a permeate pressure of 76 mmHg and a feed containing 8.8 wt% EtOH. Using 1.0 cm2 of membrane, we collected 0.25 g/hr of permeate that was found to contain 10.0 wt% EtOH.
(a) What is the permeance of our membrane to ethanol and to water?
(b) What is the expected product composition and flowrate (g/hr) for a feed containing 10.0 wt% EtOH at 60°C with operation at a permeate pressure of 100 mmHg?
- AEtOH,H2O = 1.6276
- AH2O,EtOH = 0.9232
- PEtOHsat(60°C) = 352 mmHg
- PH2Osat(60°C) = 149 mmHg

