Chapter 15: Cassava Breeding
Shui-Zhang Fei; Arti Singh; Anthony A. Mahama; and Walter Suza
Cassava (Manihot esculenta Crantz) is a dicot perennial shrub, belonging to the family of Euphorbiaceae. It is also known as tapioca, manioc, mandioca or yuca in different parts of the world. It can reach a height of 1-4 m (Fig. 1). Its tuberous storage roots are rich in starch (20-40%) and are harvested either for direct human consumption, animal feed, or industrial uses.
Cassava Anatomy
Stem
The growth habit of cassava has important implications in cassava breeding as it can affect root yield. There are two growth types:
- Erect growth type, with or without branching at the top.
- Spreading type, which is not cultivated.
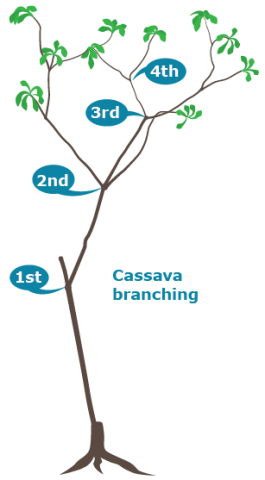
Branching occurs as a result of flower induction (Ceballos et al 2010), therefore the branchings are often called “reproductive branchings”. The branches can undergo further branching when flowering occurs, resulting in high order branchings (Fig. 2).

Roots
Cassava roots are true roots, therefore cannot be used for propagation. Cassava root is the most economically important organ of a cassava plant because of the starch-containing cells in the parenchyma tissue, the edible part of the root. Only 3-10 of the fibrous roots of a cassava plant will eventually bulk and become storage roots through secondary growth while the rest of the fibrous roots remain thin and serve to function in water and nutrient absorption (Fig. 3).

Cassava Production
Economic importance
Cassava is the sixth-most important crop in the world following wheat, rice, maize, potato and barley. It is a staple food for over 500 million people, most of whom live in developing countries where food security is a major concern. Cassava is grown successfully between latitudes of 30°N and 30°S. It is drought tolerant and can grow under annual precipitation of 600mm. Cassava can also be grown on marginally fertile soils with a pH ranging from 3-8. Its roots can be left in the ground without harvest, serving as a good food security crop in cases where other crops fail.
Worldwide production
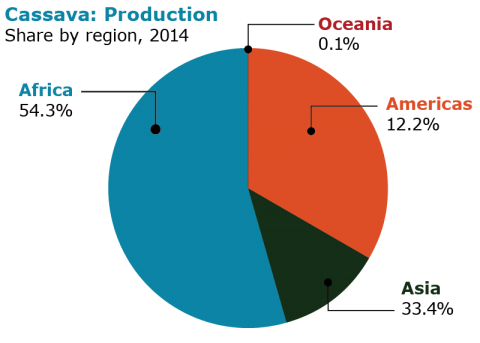
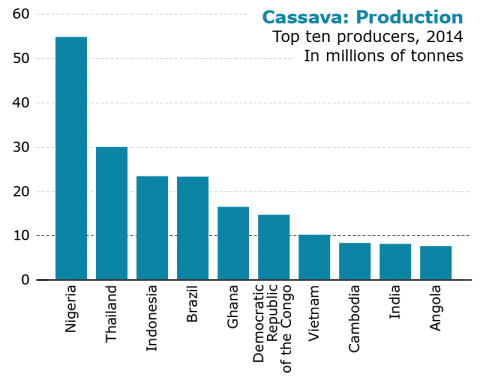
The main cassava production areas in the world are Africa, Asia, the Caribbean and South America. Fig. 4 describes the share of each main production area in 2014. Fig. 5 shows the production in tonnes in ten countries in 2014.
Main uses
Cassava roots are either directly consumed by humans as food, used as animal feed or for industrial use.
Food

Cassava roots can be eaten raw, cooked after removing the skin and rind or even baked and the charred skin removed before consumption. Cassava roots have numerous culinary uses around the world. Cassava leaves can also be consumed as a vegetable.
Animal feed

All parts of the cassava plant can be used for animal feeding. In particular, the high energy content of cassava roots makes it an ideal carbohydrate ingredient in animal diet. The majority of the cassava produced in south Asia are used for animal feed in the form of chips and pallets.
Industrial use

Cassava roots are also used for industrial purposes, primarily for extraction of starch which has a wide variety of uses. Recently bioethanol production from cassava has received great attention due to increased fossil fuel price and concerns over global climate changes caused by burning fossil fuels.
Reproductive Biology of Cassava
Flower morphology and flowering behavior
Cassava is monoecious, producing separate male and female flowers on the same plant. Flowering in cassava is highly genotype and environment dependent. While some early-flowering genotypes can flower one month after planting, others may take 24 months to flower, consequently, synchronization of flowering time can be challenging in cassava breeding programs. Flowering rarely occurs during long dry period, thus irrigation is required for crossing blocks during an extended drought.
 |
 |
Cassava inflorescence is developed from the fork of the branchings (Fig. 9). The female flowers (Fig. 10) which are larger in size but smaller in numbers than the male flowers are situated at the base of the inflorescence while the male flowers, often numerous are located on the upper part of the inflorescence.
Female flowers open 1-2 weeks earlier than the male flowers on the same inflorescence. However, male and female flowers located on different inflorescences of the same plant can still open at the same time. Consequently, selfing can occur.
Pollination behavior
Despite the occurrence of self-pollination, cassava is considered a cross-pollinated species and cross-pollination is done by insects. The average size of cassava pollen ranges from 122-148 µm, much larger and heavier than pollen of most other species. The longevity of cassava pollen is relatively short, lasting no more than 2 days.
The degree of self-pollination depends on genotype, environmental conditions, and the presence of pollinating insects. Inbreeding depression is severe in cassava, thus seedlings of selfed progeny typically exhibit low vigor and lack competitiveness.
Seed formation, dormancy, and storage

Cassava fruit is a trilocular capsule (Fig. 11), with each capsule containing a single seed (Fig. 12). It takes 75-90 days after pollination for a cassava fruit to become physiologically mature. Dehiscing of mature cassava fruits is explosive, therefore bagging must be done before fruits become mature to collect seed in controlled crosses. Freshly harvested seeds are generally dormant and a 3-6 month of dry storage at ambient temperature is necessary to break the dormancy.

Physiologically active cassava seed can germinate readily in about 15 days. The optimum temperature for germination is between 30-35°C. Cassava seed can be stored at 4-5°C and relative humidity of 60%. A germination percentage of greater than 80% has been reported for seed stored under such conditions for a year.
Propagation Methods
Cassava can be propagated by either seed or stem cuttings (stakes). Because cassava plants are highly heterozygous, seed propagation will result in highly heterogeneous offspring that no longer possess the desirable traits of the seed parent. Consequently, cassava propagation is done mostly through stem cuttings.

Multiplication rate is one of the determining factors that affect whether a new improved cultivar is successfully adopted or not. After one year, a typical mature cassava plant will produce 10-30 stakes sized at about 25cm (Fig. 13). Reducing the stake size to include only two nodes or one per stake will likely produce 100 or 200 stakes from a single plant per year, resulting a multiplication rate of 100 or 200. Such multiplication rates, although high may still not be sufficiently rapid to produce a large quantity of stakes in a short period of time to meet the consumer demand. Even higher multiplication rates have been achieved by growing 2-node stakes in high density in moist chambers and continuously removing sprouting shoots of 15-20cm long for rooting in boiled water. Rooted plantlets are transferred to soil and after a brief period of hardening are transplanted to the field for production. Such a system can produce a multiplication rate of 36,000.

Tissue culture methods have also been developed for rapidly multiplying desirable cassava germplasm. Plantlets can be regenerated from either pre-existing meristem or through somatic embryogenesis (Fig. 14). Virus-indexed plants can be obtained by culturing very small meristems and regenerating plants.
Origin of Cassava and Genetic Diversity
Cassava is widely believed to be originated from the southern rim of Amazonia. It is domesticated about 5,000-7,000 years BC (Allem, 2002) and was introduced to Africa by Portuguese and Spanish explorers, likely in the sixteenth century. Cassava did not become popular in Asia until in the 1960s.
The genus Manihot contains more than 100 species, all naturally occurring between 33°N (southwest USA) and 33°S (Argentina). Wild relatives that have been used for interspecific hybridization include M. catingae, M. dichotama, M. glaziovii, M. melanobasis and M. saxicola. Among these, M. glaziovii (ceara rubbertree) is the only species that has made significant contributions in developing cassava germplasm resistant to cassava mosaic disease.
Cassava has 36 chromosomes, forming 18 bivalents at meiosis. However, there are cytological and sequence information supporting the paleotetraploidy nature in cassava.
Breeding Objectives
Yield
Developing high-yielding cassava cultivars remains the highest priority of most, if not all cassava breeding programs. Root yield in cassava is, however, a complex trait and is affected by both genetics, environment, and their interactions. Cassava plants with an intermediate branching height have been shown to be highly correlated with high yield. Similarly, plants with good leaf retention (longer leaf life) are also found to be correlated with high root yield.
Root quality
Root quality is very important as it affects consumer acceptance and successful adoption of a cultivar. Cultivars with highly reduced cyanogenic glucosides and increased dry matter in the roots are desired. Cassava roots are naturally low in protein, therefore cultivars with enhanced protein content are desirable if they are used for animal feed. Cultivars with altered starch content and composition may be developed for specialty use.
Biotic stress

Cassava production is constrained by many biotic stresses including some of the most devastating viral diseases such as cassava mosaic disease (CMD) (Fig. 15) and cassava brown streak disease (CBD) that can cause significant yield loss. Bacterial diseases such as cassava bacterial blight and root rot can also cause damages.
Developing cassava germplasm with resistance to a number of insects including cassava mites, mealybugs, and whiteflies which are responsible for transmitting the devastating CMD is of great importance.
Abiotic stress
The shelf life of cassava roots is notoriously short, often within 2 days after harvest. This post-harvest deterioration of cassava roots is manifested by internal discoloration which causes the immediate loss of marketability (Fig. 16). Developing cassava cultivars that are resistant to this post-harvest physiological deterioration is therefore highly desirable.

Principles of Cassava Cultivar Development: An Overview
Cassava is primarily cross-pollinated, therefore individual plants are highly heterozygous. Because cassava can be easily propagated by stem cuttings, improved cassava cultivars are primarily clonal cultivars that are multiplied by stem cuttings for distribution. Therefore, the principle of developing clonally propagated cultivars for other crops also applies to cassava.
Briefly, large segregating populations are first created from which initial seedling screening is performed. Plants selected from the initial screening are then evaluated in subsequent replicated, multi-location trials that would eventually produce one or more superior clones possessing desirable traits.
The following flowchart describes the general aspects of a cassava breeding procedure:
- Population development
- Seedling evaluation and selection
- Clonal evaluation and selection
- Preliminary yield trial and selection
- Advanced yield trials and selection
- Regional trials
While the size of the initial population is large (for example, 50,000 seedlings), the number of entries is drastically reduced following each step of selection, and at the end of the regional trial, only one or a few clones may endure the rigorous selection process and are released as new cultivars.
Cassava Hybridization Techniques

Preparation of female flowers
To determine if a female flower is about to open, Kawano (1980) described a reliable method by which a petal of an unopened female flower is peeled back, if a drop of nectar is observed at the base of the pistil, the flower will open in the afternoon of the same day (Fig. 17). Female flowers ready to be pollinated are then covered with a large (20 x 25 cm) cloth bag to avoid stray pollen. Emasculation is generally not necessary because male flowers on the same inflorescence will not open until 1 to 2 weeks later when the female flowers either developed into fruits or have died.
Preparation of male flowers
Between noon and 2pm in the afternoon, freshly open male flowers are collected by glass bottle or other suitable devices and pollination can be performed immediately. A single male flower can pollinate up to three female flowers. Pollination performed after 5pm will not be as effective. Pollinated female flowers can be left uncovered to promote seed development with minimal risk of hybridization by stray pollen. However covering of the pollinated flowers with a small cloth bag is needed 1 or 2 weeks after the pollination in order to collect seeds.
Breeding Scheme

Population development
To increase the chance of obtaining a superior genotype, distinct genotypes with diverse genetic backgrounds are selected for creating the base segregating population from which evaluation and selection will be performed.
Three methods are used to create the base population:
- Controlled biparental crosses – In this case, male flowers are collected from the male parent and are used to pollinate female flowers of the chosen female parents. Seed yield from such crosses is limited because of the amount of labor involved. Many such crosses may be needed to produce a large base population. Pollination in cassava, however is viewed as one of easiest among all the crops because of its large flowers, large and sticky pollen and no need of emasculation, therefore, this method can still be productive.
- Use of crossing blocks – In this case, a set of cultivars are grown in an isolated crossing block. Male flowers are removed from all cultivars that are chosen to be female parents and seed harvested from the female parents are hybrid seeds that can be used to start the selection process. The physical separation of male and female flowers in a cassava plant makes it easy to remove male flowers. This method is commonly used in recurrent selection.
- Polycross – In this case, each elite parent is replicated several times and they are randomly grown in a polycross nursery. Random pollination among parents ensure a fair representation of each parent in the progeny. This method is more efficient in producing sufficient quantity of hybrid seed than the biparental method. However, it does not prevent self-pollination.
Seedling evaluation trial (Year 1)
Once a base population is created using one of the methods described earlier, selection can immediately start. Up to 100, 000 hybrid seeds are directly sown in the field and seedlings will be screened for resistance to major disease such as cassava mosaic disease, cassava brown streak disease and for ideal growth habit, i.e., branching at a medium height (100cm for example) and low hydrogen cyanide (HCN) content in leaves. At harvest, additional screening is performed for plants producing compact roots with short necks.
In this step, selection is concentrated on eliminating poor genotypes rather than selecting for good genotypes. A total of up to 3,000 individuals may be selected for further testing.
Clonal evaluation trial (Year 2)
Entries selected from the seedling evaluation trial are multiplied by stem cuttings and each entry is grown in single-row plot without replication. Resistance to major diseases is again screened. In addition, root yield, root dry matter content as well as the HCN content in the root of each entry is evaluated. A locally-adapted leading cultivar is grown as a check to aid selection. A total of up to 100 individuals are selected.
Preliminary yield trial (Year 3)
Stem cuttings from each of the selected entries are grown into larger plots that are replicated at least twice, along with a locally-adapted check cultivar to evaluate yield potential. This trial is conducted in multiple locations. The clonal entries within each location is randomized. Besides root yield, root quality and resistance to major disease and other pests will be continuously monitored. Evaluation of consumer acceptance is conducted at this stage. A total of 25 genotypes may be selected at the end of the trial.
Advanced yield trial (Year 4)
Stem cuttings from the selected genotypes are grown into larger plot size with more replications (for example 4) and more locations, along with a locally-adapted check cultivar. Selection is primarily focused on root yield and root quality traits. At the end of the trials, up to 5 genotypes may be selected.
Regional yield trial (Year 5)
At this stage, the best clones are grown on large-scale farms in multiple locations within the target region. At least four replications are used within each location.
Final selection will be made based on data for yield, root quality, and consumer acceptance. Planting materials will be rapidly multiplied and distributed to end-users.
Note that “upgraded” base populations can be continuously created by crossing elite entries selected at the end of each step and additional germplasm from other sources. A new cycle of selection can then be performed on the “upgraded” base populations.
Farmer Participatory Trial
Traditionally, farmers are not involved in the breeding process until a new cultivar is close to being released commercially, therefore farmers have limited influence on how new cultivars are developed.
Participatory Plant Breeding (PPB) is a process in which farmers play an active role early in the breeding process and it has become increasingly important in breeding grain crops as well as vegetatively propagated crops such as cassava. For example, in developing early bulking cassava cultivars in Kenya, farmers are invited to participate in the selection process after the 1st clonal evaluation trial. Farmers evaluated root quality traits including appearance, size, taste/texture, and their inputs were used along with those of the breeders in making final selection decisions. A similar effort involving farmers early in the breeding process was also reported in developing cassava mosaic disease-resistant cultivars in Ghana.
Subsistence farmers in Africa often grow multiple crops/cultivars to counter the uncertain and adverse climatic conditions. Under such circumstances, PPB has been proven very useful and it also increased the adoption rate of new cultivars released by programs in which farmers have actively participated in selecting.
Germplasm Conservation Centers and Practice
Genetic diversity is essential to plant breeding. Modern agricultural practices and the destruction of native cassava habitats in the centers of diversity cause significant erosion of cassava genetic resource. Therefore, collection and conservation of cassava germplasm is of paramount importance to sustained successes in cassava breeding. Great efforts have been put forth to collect and conserve cassava germplasm. Table 1 lists the number of accessions maintained at each location. The international institutes, the Centro Internacional de Agricultura Tropical (CIAT), Colombia, and the International Institute for Tropical Agriculture (IITA), Nigeria, both of which hold the UN mandate for cassava maintain a large number of accessions. These accessions are available freely to the public.
Cassava germplasm is typically conserved in one of the three methods:
Field genebanks
Cassava plants representing each accession are grown and maintained in the field. As is practiced at IITA, eleven plants of each accession are grown on a 2.5m row plot with a 25cm space between plants and 50cm space between rows. Field genebanks require a large amount of field space and germplasm may be lost due to various biotic and abiotic stresses.
Seed genebank
Seed of each accession are maintained in controlled environment with low temperatures and humidity. It has been reported that cassava seeds remain viable after 14 years of hermetic storage at -20°C with 6% moisture content in the seed. As is practiced at IITA, seeds representing each accession are harvested in bulk from all plants of an accession and are therefore heterogeneous.
In vitro genebanks
| Location | Number of accessions | Type of accession (%) | ||||
|---|---|---|---|---|---|---|
| Wild species | Landraces/ old cultivars | Research materials/ breeding lines | Advanced cultivars | Others/ mixture | ||
| CIAT | 5436 | 1 | 87 | 11 | 0 | 1 |
| Brazil | 2889 | 0 | 0 | 0 | 0 | 100 |
| IITA | 2756 | 0 | 28 | 47 | 0 | 25 |
| India | 1327 | 0 | 0 | 0 | 0 | 100 |
| Nigeria | 1174 | 0 | 0 | 0 | 0 | 100 |
| Uganda | 1136 | 0 | 4 | 89 | 7 | 0 |
| Malawi | 978 | 0 | 22 | 72 | 6 | 0 |
| Indonesia | 954 | 0 | 0 | 0 | 100 | 0 |
| Thailand | 609 | 0 | 0 | 100 | 0 | 0 |
| Benin | 600 | 0 | 100 | 0 | 0 | 0 |
| Togo | 435 | 0 | 100 | 0 | 0 | 0 |
| Other | 14148 | 6 | 26 | 3 | 14 | 51 |
In vitro cultures established from apical buds or nodes containing axillary buds are maintained on culture media or culture conditions that slow down the growth of the culture. Such cultures can be maintained for 8-12 months without the need for subculture. Following disease and virus indexing, these cultures are suitable for exchange among collaborators across countries.
Cassava Genetics
Marker-assisted breeding in cassava
DNA markers are variations in DNA sequences, and such variations among individuals can be easily detected using polymerase chain reaction (PCR) or high throughput sequencing technology. DNA markers, unlike phenotypes are not affected by environment or developmental stages; therefore they can be assessed at any time and from any plant tissue. They are used for cultivar fingerprinting, assessing genetic diversity or marker-assisted selection for traits for which markers are available.
In cassava, many simple sequence repeat (SSR) and single nucleotide polymorphism (SNP) markers have been developed. Recently, a high-resolution composite map covering 2412cM and organizing 22,403 genetic markers on 18 chromosomes has been described for cassava (International Cassava Genetic Map Consortium). This map will greatly facilitate marker-assisted selection in cassava, particularly for selecting plants resistant to the cassava mosaic disease for which linked DNA markers have been developed.
Sequencing of the cassava genome
The genome size of cassava is estimated to be ~770Mbp. It has recently been sequenced from a partially inbred line AM560-2 developed at CIAT. The complete sequence is made available by the Joint Genome Institute (JGI). A resequencing project in 2012 report a genome size of 760 Mbp. The sequenced genome will undoubtedly enable a whole array of research aimed at improving the crop.
Application of Biotechnology in Cassava
Traditional plant breeding is a very long and imprecise process and it could take more than a decade to release an improved new cultivar. Genetic transformation, however can introduce a trait very efficiently and rapidly. Furthermore it can break the reproductive barrier and transfer traits from unrelated species to cassava. Because cassava is a vegetatively propagated species, traits introduced into an existing elite cultivar or a farmer-favored landrace can be “fixed” immediately without the need of inbreeding or backcross as is the case in sexually propagated species.
Successful genetic transformation has been accomplished in cassava using either Agrobacterium or particle bombardment. The key to the success relies on an efficient plant regeneration protocol. The current standard practice is to use the friable embryogenic callus (FEC) derived from immature leaf explants cultured in vitro for gene transfer.
The potential of using a transgenic approach to produce novel cassava germplasm has been demonstrated in transgenic lines with insect and disease resistance, herbicide tolerance, altered starch content and increased protein level as well as reduced cyanogenic content in cassava storage roots. Another noted example is the biofortified cassava generated by the BioCassava Plus (BC+) program supported by the Bill and Melinda Gates Foundation. These biofortified cassava varieties demonstrated the potential of developing cassava as a more nutritionally complete crop with increased zinc, iron, proteins and vitamin A.
Major accomplishments in cassava breeding
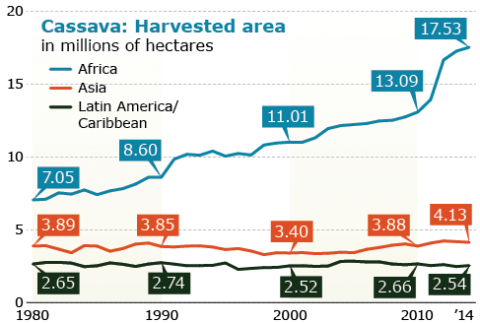
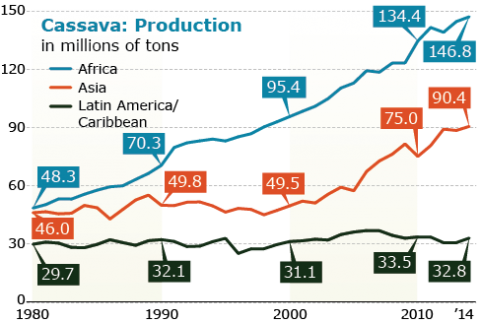
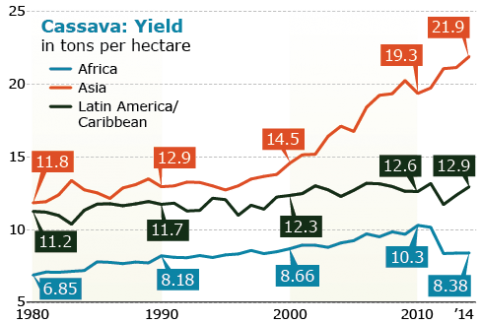
Notable is the consistent increase in production and harvested area despite the low yields in Africa compared to Asia and Latin America/Caribbean (Figs 19, 20, 21). Thus there is potential for even greater increases in Africa with the increased availability of improve cultivars.
References
Allem, A.C. 2002. The origins and taxonomy of cassava. In: R. J. Hillocks, J. M. Thresh and A. C. Bellotti, editors, Cassava: Biology, Production and Utilization. CABI Publishing. p. 1-16.
Ceballos, H., E. Okogbenin, J.C. Pérez, L.A.B. López-Valle, and D. Debouck. Cassava 2010. In J. E. Bradshaw editor, Root and Tuber Crops, Handbook of Plant Breeding. Springer New York Dordrecht Heidelberg London. p. 53-96.
FAOSTAT. https://www.fao.org/faostat/en/#data/QCL/visualize
How to cite this chapter: Fei, S., A. K. Singh., A. A. Mahama, & W. Suza. (2023). Cassava Breeding. In W. P. Suza, & K. R. Lamkey (Eds.), Crop Improvement. Iowa State University Digital Press.

