Chapter 3: Genetic Variation and Germplasm Usage
Asheesh Singh; Arti Singh; Jessica Barb; Anthony A. Mahama; and Walter Suza
The presence of genetic variation is a key prerequisite for genetic improvement in plant breeding and plays a pivotal role in germplasm usage in breeding programs. Therefore plant breeders and students in plant breeding can benefit immensely from an understanding of sources of genetic variation present, and ways of creating genetic variability where it is limited. The source of genetic material in a breeding program may come from one’s own breeding program, a colleague’s breeding program with the same or different organizations, or gene banks, among others. Good stewardship needs to be followed by plant breeders to utilize the genetic material.
- Know processes that create genetic variation
- Gain an understanding of the concepts of types and origin of genetic variation
- Become familiar with plant genetic resources and working with variability in hybridizations
- Know the legal issues with germplasm usage and exchange
Relationship of Plant Breeding to Natural Selection
Creating Genetic Variability
Natural selection requires three main processes to function:
- Processes that create genetic variability: gene mutation, recombination, chromosomal segregation, gene flow are some of the ways to create genetic variability. This provides the potential to change the composition of individuals in the population. Mutations are considered random as they are not created to address a “need” of the organism. Therefore mutations can be neutral, harmful, or beneficial. Somatic mutations (occurring in the non-reproductive cell) are not useful to genetic variability. Gene flow can be an important source of genetic variation if genes are carried to a population where those genes did not previously exist (Fig 1).
- Processes that rearrange genetic variability: natural selection or random genetic drift. These processes will lead to a change in population, due to the favoring of reproduction of certain individuals over others, thus causing a change in the gene pool (genes possessed by the mating population). Natural selection operates through reproductive fitness (the ability to produce offspring that contribute to the gene pool of the next generation).
As the term implies, random genetic drift is random and uncontrollable. For example, in a population, some individuals may leave more offspring by chance than other individuals. Let us consider a hypothetical situation in a forest where there are 50% each of two tree species. Species A is predominant in the western part of the forest and species B is predominant in the eastern part. If fire destroyed 80% of trees in the western part of the forest, species A will be significantly reduced in number, and so species B will leave more offspring, leading to a genetic drift. It is important to note that preponderance of offspring of species B is due to the chance destruction of species A, and not necessarily because species B is healthier or more productive. Unlike natural selection, genetic drift is neutral to adaptation. In the forest fire example above, if species B had wood properties that made them fire-resistant (remember this is a hypothetical example) then a fire will destroy species A and reduce the number of species A offspring in the next generation compared to species B. Because this trait of fire protection is genetic, after repeated fires, species B will have more off-springs and will evolve due to natural selection. This example can be extended to a crop plant and disease.
- Processes that maintain the product (minimize disturbance).
These processes or mechanisms serve to protect the integrity of a population’s gene pool. This functions to maintain the genetic identity of the product, for example, due to reproductive isolating mechanisms. Examples of reproductive isolating mechanisms include sterility or failure of mating due to asynchrony (where males flower and shed pollen before the stigma of the females are receptive or vice versa).
Deliberate Choice
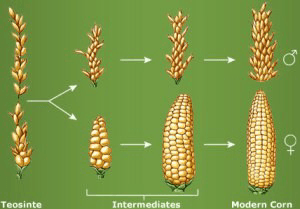
Artificial selection describes the deliberate choice of individuals for breeding in each generation and the advancement of select individuals. Directional selection is a form of artificial selection in which phenotypically superior plants are chosen for breeding. Artificial selection has been practiced for thousands of years by humans to make improvements in plant species. For example, artificial selection led to the rise of modern maize from its progenitor, teosinte (Fig. 2). Numerous studies show that teosinte (Zea mays ssp. parviglumis, a grass species) is a progenitor of maize (Zea mays L. ssp. mays). Very small differences in morphology (under genetic control) differentiate maize and teosinte. For example, teosinte has a cupulate fruit case protecting each kernel and the rachis segment (internode) and glume (modified bract) cover the kernel (Fig. 3). The cupule and glume are present in maize but they are significantly reduced in size and therefore do not surround the kernel. In maize, these organs form the cob. Ears of teosinte disarticulate at maturity such that the individual fruit cases become the units of seed dispersal. Ears of maize remain intact at maturity, which allows for easy harvest by humans. In teosinte, each cupulate fruit case holds a single-spikelet (kernel-bearing structure). In teosinte, the cupulate fruit cases are borne in two ranks on opposite sides of the longitudinal axis of the ear. In maize, the cupules are borne in four (or more) ranks.
Recreating Primitive Maize
Previous work by George Beadle has shown that primitive maize can be recreated by crossing teosinte and modern maize. While some of the changes between teosinte and maize may have happened naturally, the rest resulted from domestication and artificial selection as these differences made maize suitable for production for humans. Today we continue to improve the yield of maize using directional selection.

Making Progress
Artificial selection in genetically heterogeneous populations always leads to a successful outcome (i.e., mean change in population phenotype over generations in the direction of selection). This is true unless a biologically constraining limit is reached. The mean of a trait can be altered in both directions (i.e., an increase or a decrease in a trait’s arithmetic mean value) if genetic variability exists in a population.
Genetic variation is ESSENTIAL for making progress using artificial selection.
Can we change the mean phenotype of a genetically uniform population (or completely inbred genotype) over generations?
What will happen if mutations occur in the genetically uniform population? Will the mean phenotype change over generation in the same genetically uniform population (now with mutations)?
Successful Maize Experiment
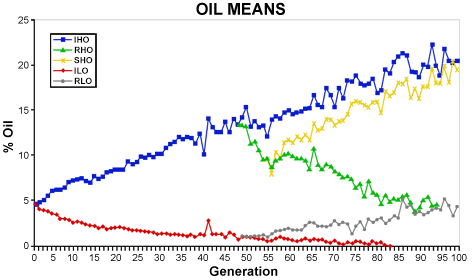
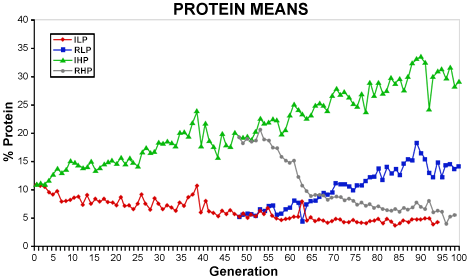
In 1896, C.G. Hopkins started long-term artificial selection experiments looking at oil (Fig. 4) and protein (Fig. 5) content in maize. The open-pollinated corn cultivar Burr’s White was used as the founder population. Four strains were established: Illinois High Oil (IHO), Illinois Low Oil (ILO), Illinois High Protein (IHP), and Illinois Low Protein (ILP) with high and low referring to the direction of the selection. After 48 generations, reverse selection was started in each strain to establish the Reverse High Oil (RHO), Reverse Low Oil (RLO), Reverse High Protein (RHP), and Reverse Low Protein (RLP) strains. After seven generations of selection in RHO, selection was again reversed to create the Switchback High Oil strain (SHO) to study the effect of selection.
Protein Content
The effects of selection on oil content ceased (i.e., Generation 85) in the ILO strain when the oil content reached a level that was no longer measurable with the analytical tools used in this experiment. Protein content reached a lower limit after approximately 65 generations, likely due to biological (i.e., physiological) limit in this crop species. An upper limit was not reached for oil content in IHO and SHO indicating that significant genetic variance still existed in these strains even after 100 generations of selection.
Overview of the Plant Breeding Process
Flow Chart
Figure 6 presents a broad outline of plant breeding process. For a detailed flow chart on breeding process see Simmonds, 1979.
The three main phases of the plant breeding process are:
- Germplasm development: Generally one trait is improved at a time. Crossed are made between wild accessions or related species and/or between elite breeding line or cultivar.
Considerations on commercial suitability is low or non-existent. The intention of the work is to develop improved parental germplasm, not a cultivar. Genetic conservation and genetic variability is improved. Genebanks are more heavily relied on for parental stock material. - Cultivar development: Generally several traits are improved simultaneously. The finished product is a genotype or population that has desirable characteristics for release as a cultivar. Crosses are made between elite lines as parents and may include a germplasm line (see above) as one of the parents. In general, both parents are elite lines (See Steps in Cultivar Development). Considerations on commercial suitability are primary. The intention of the work is to develop an improved cultivar to be grown by farmer(s). Breeder will make phenotypic and genotypic selection decisions on multiple traits and in several generations (pure-bred and inbred lines). Wide adaptation and performance testing is done prior to commercialization.
- Technology Transfer: For germplasm development, there are smaller components of technology transfer for scientists and breeders. For cultivar development, there is a larger component of technology transfer for scientists, breeders, agronomists, pathologists, entomologists, seed merchants, and extension scientists.

Cultivar Development Strategies
In the public and private sectors, the same individual may be responsible for germplasm development and cultivar development phases. In other situations, two or more individuals may be engaged in these two phases independently but collaboratively within the same or different teams. Germplasm developers will be more interested in working on one or few traits (to transfer them from unadapted or wild relatives) and would not be as concerned about its overall suitability for a fit into a commercial release market. On the other hand, a cultivar development breeder has to consider the commercial requirements of his/her crop and its overall suitability.
In the cultivar development strategies, elements that are common in all programs are:
- Setting objectives
- Identifying available parents?
- Creating breeding populations
- Evaluating and selecting in these population in appropriate environments to meet the objectives
- Identifying the most suitable genotype for commercial release
Setting Breeding Objectives
Definition
Breeding objectives are based on a mandate (market segment needs), organizational focus, farmer requirement, industry needs, profitability, and sustainability. Objectives need to be clearly defined and based on importance, feasibility and cost-effectiveness. It is not sufficient to set an objective as ‘increase yield’. The breeder should put some quantifiable description, such as “increase yield by x% over check ABC”, where the comparison has to be made head-to-head. Plant breeding is an expensive activity and careful consideration needs to be made prior to setting objectives. The breeding team needs to engage growers, industry, and consumers to decide on objectives. In a large company, this may be done by a different team and the results communicated to the breeder to help her/him define the objectives. A breeder may develop the highest yielding inbred or hybrid parents or population, but without growers’ ability to grow it, this product (inbred, hybrid, population) will not be a commercial success. For example, if a very high yielding genotype has poor storability, growers and industry will not accept this genotype. In plant breeding, multiple objectives are generally set and a prioritization made to decide on ‘must to have’ versus ‘nice to have’ trait. ‘Must to have’ are traits that absolutely need to be included in the product (pure line, or hybrid, or OPV) for it to be suitable for commercial release, whereas ‘nice to have’ are traits, which are not essential but may add value to the product.
Identifying Parents
An important consideration for setting breeding objectives is to identify parents for hybridization that have the necessary traits that the breeder will want in the cultivar to be developed. Sources of parental material will be genotypes or populations from your own program, your colleagues’ programs (within or outside of your organization), international breeding centers, and gene banks. We will learn a little more about sources of parental material in the next few sections.
After setting of objectives, a breeder will create breeding populations (i.e., create genetic variability) by crossing two or more parents. In crop species with sexual reproduction, generation advancement is generally occurring in parallel with selection for traits as per defined objectives. Once a finished product (genotype) is ready, broader adaptation testing is performed prior to picking the most suitable cultivars for commercialization.
In the next section, we will learn about gene banks, which contain accessions that may be useful to a breeder as sources of genetic variability for use in breeding.
GeneBanks: Role, Procedures, Acquisition, and Stewardship
Roles of Gene Banks
For decades, local, regional, and international efforts have been attempting to preserve valuable agrobiodiversity for future generations by setting up collections of genetic resources, called genebanks. Genebanks contain ‘landraces’ or local varieties of cultivated and non-cultivated wild relatives. This serves to protect and preserve seed diversity as well as provide an accessible source to plant breeders to obtain seed of interest. There are currently about 1,750 institutional crop collections around the world, as well as a number of community-based seed bank initiatives. CGIAR Research Program for Managing and Sustaining Crop Collections is dedicated to maintaining the 706,000 samples of crop, forage, and agroforestry resources held in “genebanks” at 15 CGIAR research centers around the world. Species which include cereals, legumes, roots and tubers, trees, and other essential staple crops are stored in CGIAR international collections. All accessions within these collections are for the international public good, available under the terms and conditions negotiated by the International Treaty on Plant Genetic Resources for Food and Agriculture.
In the USA, the National Plant Germplasm System aids scientists and addresses the need for genetic diversity by:
- acquiring crop germplasm
- preserving crop germplasm
- evaluating crop germplasm
- documenting crop germplasm
- distributing crop germplasm
GRIN
For example, for the USDA’s Germplasm Resources Information Network (GRIN), the steps are to search for genotypes that you are interested in and then place an order to receive seed:
- Access GRIN’s Seach Query interface.
- Access GRIN’s Order Form.
The breeder should determine which genebank has the collection of material in their crops, proceed to search the genebank and order seed. This process involves numerous paperwork (agreements, seed importing or exporting permits and customs documents) and planning ahead is critical to ensure that you receive seed on time.
[Note: Many times it is useful to contact the curator or other scientists at a genebank as they can sometimes help to make suggestions on a specific trait or accession you may be looking to obtain. However, one needs to do their groundwork first.]
Type of Variability and Sources of Genetic Material
Natural Variability – The Gene Pool Concept
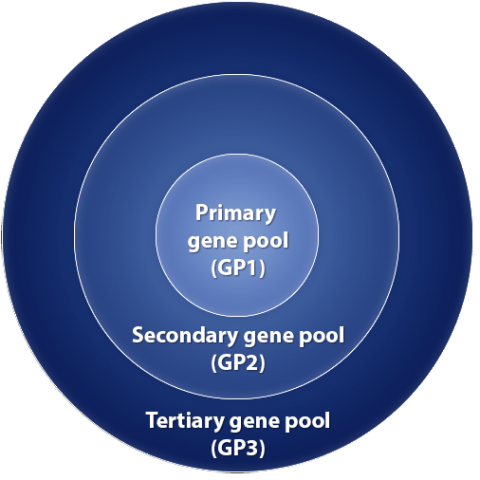
For plant breeders, it is very important to be aware of available germplasm resources that will be useful to improve traits. The gene pool concept was proposed by Harlan and de Wet (1971) as an attempt to provide a practical guide to place existing classifications into genetic perspective. This information on the relatedness among crop plants and their relatives could be useful to breeders and geneticists wishing to make crosses among them. It is important to note that the gene pool concept did not attempt to change the taxonomy. Its purpose is to serve as a guide to plan breeding activities. Various genetic resources are assigned to different gene pools of a crop species based on ease of hybridization, i.e., ability to move genes between them. The three major gene pools are: primary, secondary and tertiary. Gene pools are not static but change as more information becomes available or as new technologies become available to manipulate genomes. For example, in their paper soybean was reported not to have a secondary or tertiary gene pool. However, we now consider that 26 perennial Glycine species are in tertiary gene pool and G. tomentella has now been used to transfer genes to G. max (Singh et al. 2014; R.J. Singh, USDA-ARS, IL, personal communication). Therefore breeders need to be aware of what is going on around them with the use of unique genetic material.
Primary Gene Pool
Species in primary gene pool can be cultivated, landraces, farmer developed or maintained population, ecotypes, and spontaneous races (wild or weedy). Among forms of this gene pool, crossing/gene transfer is easy; hybrids are generally fertile (i.e., no sterility issues) with normal chromosome pairing and gene segregation. Most breeders work exclusively within this gene pool which is also the major source of genetic variation for improvement programs. Remember that most breeding programs that are engaged in developing cultivars for commercial production work on elite material exclusively and would spend very little direct efforts on unadapted or wild relatives (because of undesirable linkage blocks, breaking of desirable linkage block and epistatic interactions with undesirable genes from wild relatives).
Secondary gene pool
Crop’s secondary gene pool will include species between which gene transfer is possible, but difficult. Hybrids tend to be sterile; chromosomes pair poorly during meiosis; F1 plants are weak and develop to maturity with difficult; because of some sterility in F1’s , recovery of desired types in advanced generations is generally difficult. The secondary gene pool includes related species within the same genus, although all species within a genus won’t be in the secondary gene pool and it is also possible that species outside the genus can be in this gene pool.
Tertiary Gene Pool
Gene transfer between a crop and a species in its tertiary gene pool is very difficult (will require embryo rescue, chromosome doubling, bridging species to obtain hybrids). This gene pool includes distant relatives in other genera or distantly related species within the same species. Hybrid sterility is common, although chromosome doubling may restore fertility by providing homologues for each chromosome. The boundaries of this group are poorly defined and shift as new hybridization techniques are developed.
Hybridization
A bridging species is a third species that facilitates exchange of germplasm between the other crop species and tertiary gene pool species by developing complex hybrids. In their paper, Harlan and de Wet (1971) described a classic example of the use of bridging species where there was an interest to cross Elymus x Triticum. As expected, hybrid seed could not be obtained. When embryo rescue was used, very few hybrids were obtained and even then these were sterile. However, these researchers found out that if they used Agropyron x Triticum derivative as female parent and then crossed the hybrid to Elymus, introgression of Elymus alleles was possible without need for special technique (See Harlan and de Wet, 1971).
Wide Hybridization or Interspecific/Intergenetic Hybridization
‘Wide cross’ refers to crossing that involves individuals outside of cultivated species. This typically involves the secondary and/or tertiary gene pools. Even though it is difficult, it may be useful to transfer vitally important traits, including disease resistance, or other traits simply not found in cultivated genotypes. Many examples exist in wheat and rice.
Examples
Example 1: In wheat, the T1BL.1RS wheat (Triticum aestivum L.) – rye (Secale cereal L.) has been of particular interest and was widely used in bread wheat breeding programs worldwide. At one point, it was estimated that several million hectares of wheat were planted to cultivars possessing this translocation (tertiary gene pool: rye to wheat crop). This segment had disease resistance cluster for leaf rust, stem rust, stripe rust and powdery mildew, all of which are important diseases of wheat. Additionally, this segment was reported to possess genetic factors that improved grain yield and kernel weight. Resistance to specific genes in the translocation segment have been overcome in some parts of the world, which shows the continual nature of plant breeding where better genetic packages (cultivars) need to be developed.
Example 2: In rice, the first example of transfer of a useful gene from wild species was the introgression of a gene for grassy stunt virus resistance from Oryza nivara to cultivated rice. Other examples are transfer of Xa-21 for bacterial blight resistance from O. longistaminata to cultivated rice; CMS sources from O. perennis and O. glumaepatula into rice for hybrid rice production. In 1970’s, grassy stunt virus epidemics were reported in several countries and this was transmitted by brown plant hopper (diseased rice plants produced no panicles or small panicles with deformed grains) leading to severe yield losses. Several thousand accessions of cultivated rice and wild species of Oryza were screened for resistance, which identified one O. nivara accession as resistant. Plant breeders then successfully transferred grassy stunt virus resistance to improved varieties through a backcross breeding method and resistant varieties were released for cultivation. Other examples include, transfer from O. officinalis into elite rice genes for resistance to brown plant hopper, white backed plant hopper (WBPH) and bacterial blight. Some other examples are presented Table 1.
| Trait transferred to O. sativa (AA Genome) | Donor Oryza Species | |
|---|---|---|
| Wild species | Genome | |
| Grassy stunt resistance | O. nivara | AA |
| Bacterial blight Resistance | O. longistaminata | AA |
| O. officinalis | CC | |
| O. minuta | BBCC | |
| O. latifolia | CCDD | |
| O. australiensis | EE | |
| O. brachyantha | FF | |
| Blast resistance | O. minuta | BBCC |
| Brown planthopper resistance | O. officinalis | CC |
| O. minuta | BBCC | |
| O. latifolia | CCDD | |
| O. australiensis | EE | |
| O. granulataa | GG | |
| Whitebacked planthopper resistance | O. officinalis | CC |
| Cytoplasmic male sterility | O. sativa f. spontanea | AA |
| O. perennis | AA | |
| O. glumaepatula | AA | |
| Yellow stemborer resistance | O. brachyanthaa | FF |
| O. ridleib | HHJJ | |
| Sheath blight resistance | O. minutia | BBCC |
| Tungro tolerance | O. rufipogona | AA |
| O. rufipogona | AA | |
| Increased elongation ability | O. officinalisb | CC |
| Tolerance to acid sulfate soils | O. rufipogona | AA |
| O. rufipogona | AA | |
| O. rufipogona | AA | |
Artificially Created Variability: Mutation and Transgenes
Induced Mutation to Augment Genetic Diversity
Novel genes are produced by several methods, commonly through the duplication and mutation (Fig. 8) of an ancestral gene, or by recombining parts of different genes to form new combinations with new functions. Lethal mutations do not carry their germline forward, however, nonlethal mutations accumulate within the gene pool and increase the amount of genetic variation. The abundance of some genetic changes within the gene pool can be reduced by natural selection, while other “more favorable” mutations may accumulate and result in adaptive changes. A germline mutation gives rise to a constitutional mutation in the offspring, that is, a mutation that is present in every cell.
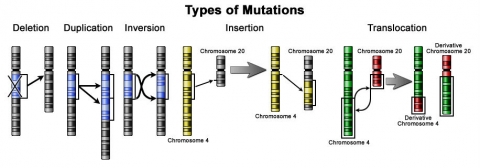
Mutation Breeding
Deletions lead to loss of gene(s) and duplication can lead to an additive effect due to added gene(s). In inversion, linkage block changes occur and other genes in close proximity will co-segregate. In insertion and translocation, a gene moves to a new chromosome, and can have similar effect as duplication.
[Note: Mutations can be subdivided into germ line mutations, which are passed on to descendants through their reproductive cells, and somatic mutations, which involve non-reproductive system cells and are therefore not usually transmitted to descendants].
Examples of Success with Mutation Breeding
Quality Protein Maize (QPM). Maize endosperm protein is deficient in two essential amino acids, lysine and tryptophan. The opaque 2 mutant gene, together with endosperm and amino acid modifier genes, was used for the development of QPM varieties. Compared to regular maize, QPM has about twice as much lysine and tryptophan, and 30% less leucine, which makes it suitable and useful for human and animal nutrition. QPM varieties are now estimated to be grown on millions of hectares. The high protein content and better amino acid profile is achieved by “opaque-2” single gene mutation. In the early 1960s, a mutant maize with similar total protein content but double the amount of lysine and tryptophan was developed. Subsequent conventional breeding efforts generated numerous cultivars with improved agronomic characteristics, and these were referred to as QPM. Dr. Evangelina Villegas and Dr. Surinder Vasal were awarded the ‘World Food Prize’ in 2000 for their work on development and advancement of QPM cultivars in the world.
Approaches
If the goal of mutation breeding is to alter only a single trait, the plant breeder needs to be aware that other regions of the genome (i.e., other genes) may have been mutated and also that, that one change may alter other aspects of the plant. Hence extensive agronomic testing of that single mutant is required prior to commercialization or extensive use as a parent in the breeding program.
Traditionally, chemical or physical agents were used to induce mutations in crop genomes, and included radiation (X-rays, gamma rays, fast neutrons, etc.), chemicals such as ethyl methane sulfonate (EMS) and others. These mutagens can disrupt chromosomes, causing deletions, insertions, breakage, etc., and will create genetic variation. Major disadvantage of this approach is the non-targeted mutation events. After receiving your M1 seed (one has to send several thousand seed of the same cultivar) plant breeder has to increase the generation to achieve homozygosity (mutant allele will segregated initially) and constantly phenotype for the traits of interest. This can be very resource intensive depending on the cost to phenotype the trait of interest (field for morphological trait or lab for quality trait or chemical component). At low doses, chromosomal changes are not as dramatic (it is desirable not to use high doses as major chromosomal aberrations and lethality can occur) and the mutation frequency is low, therefore warranting large population sizes to be screened. This leads to high expenses to phenotype and sometimes very difficult to identify a target mutant event.
Some of the newer approaches include, space light ion irradiation, use of restriction endonucleases, Zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas-based RNA-guided DNA endonucleases. These techniques lead to genetic modifications by inducing DNA double-strand breaks that stimulate error-prone nonhomologous end joining or homology-directed repair at specific genomic locations leading to site targeted mutation.
Procedure
The procedure to create a mutant population is briefly as following: Seed (M0) of an inbred (homozygous, homogenous) genotype is subjected to treatment (chemical, physical, etc). The treated seed are then grown and plants grown from these seeds (treated with a mutagen) form the M1 population (where M stands for Mutant and ‘1’ refers to first the generation in development similar to the concept of filial generation we learned in previous chapter). It is important to realize that although we started from an inbred line, after seed treatment the resultant M1 generation will consist of plants that are heterogeneous, as each M1 plant may have different mutation (or several mutations) in its genome. Each plant will also be heterozygous (or hemizygous) at numerous loci. This would mean that M1 is similar to an F1 developed from noninbred parents. The step includes, self-fertilization of the M1 plants to develop the M2 generation. This will allow the recessive mutants to be made homozygous in order to produce observable phenotypes. Identifying mutants generally requires large populations of M1 plants. Other considerations are size of M1 and M2 family to recover the mutant type, ploidy level, genetically effective cell (GEC) present in germline (these are those cells of the germline that contribute towards formation of gametes and so to offspring), frequency of chimeras. After several M generations, mutant phenotype is confirmed and established as stable (non-segregating). Mutant line then can be used in a forward or backcrossing program to transfer the favorable mutant to elite cultivars using multiple but separate crosses to ensure recovery of the elite phenotype together with the mutant trait.
Transgenic Approaches to Increase Genetic Diversity
Transgenic technology involves the transfer of cloned genes via transformation or particle bombardment so that the transformed plant expresses the foreign gene. Theoretically, the genes can come from virtually any living organism – from within the same primary gene pool to beyond the tertiary gene pool. Over the past few years, mainly only single genes have been transferred, such as herbicide tolerance in various crops, or corn borer resistance in corn.
Creating Breeding Populations – Types of Crosses
Types of Crosses
Once objectives are set and the breeder has done background investigation to pick the parents that possess the traits that will help meet the objectives, she/he will move to the next step in plant breeding process, which is to develop breeding populations. While some breeding population may start from landraces, most populations will be made by making planned hybridizations (crosses). The primary purpose of crossing is to expand genetic variability by bringing together genes from the parents in the cross to produce offspring that contain genes that will help meet the objectives. Sometimes, multiple crosses are made to generate the variability in the base population to begin the selection process in the program. In most self and cross pollinated species where the product is an inbred line or a hybrid, single crosses are made, while complex crosses are made in population improvement schemes. Parents are selected to have the maximum number of desirable traits and minimize undesirable traits (what is generally called an ‘elite by elite’ cross). This way, recombinants that possess both sets of desirable traits will occur in significant numbers in the F2, the generation of maximum variability (in self-pollinating and inbred line development programs). Several factors will impact the population size include number of genes differing among parents in a cross, number of alleles per locus, and linkage of the gene loci.
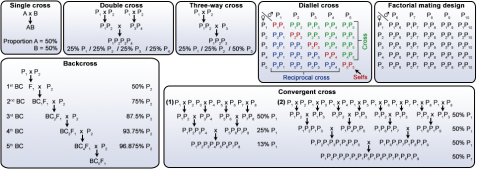
Major types of crosses made
- Single cross. This is attempted when a breeder is making a cross between two elite lines. (Line A x Line B).
- Three-way cross. If two lines are not sufficient to bring together all the necessary traits to meet the objectives, multiple cross with three parents can be used to provide an opportunity to obtain recombinants with all the desirable traits. Three way cross is (Line A x Line B) x Line C. If cultivar development is targeted in a three way cross, the third parent ‘Line C’ in our example, should be an elite and adapted genotype to get at least 50% of favorable genetics.
- Double cross. A double cross is a cross of two single crosses [(Line A x Line B) x (Line C x Line D)]. The breeder should attempt to make the two single crosses in the same season and then cross the resultant F1 in the next season crossing cycle to minimize the time to obtain F1 from the double cross. More parents start to introduce more opportunities to break linkages (including favorable ones) and there is a low frequency of obtaining desirable recombinants in the early generation selection. The double-cross hybrid is more genetically broad-based than the single-cross hybrid.
- Diallel or Partial Diallel cross. A diallel cross is one in which each parent is crossed with every other parent in the set (complete diallel), yielding n * (n – 1)/2 different combinations (where n is the number of entries) excluding reciprocal crosses where the female/male order is reversed. For example, where n equals 9, 36 crosses will be made.This method requires making a large number of crosses and it is more suitable for cross-pollinated species. This method is generally used for genetic studies and not for population development. Sometimes, a partial diallel is used in which only certain parent combinations are made. For n equals 9 example, not all 9 parents will be used in crosses.
- Back cross. The primary goal of this crossing method is to incorporate a specific trait (from a donor parent) into an existing elite cultivar (referred to as recurrent parent in back crossing). The donor parent has one trait that a breeder desires to include into an elite cultivar, which is considered to possess all necessary traits except this one trait. The resultant product (after successive crossings to recurrent parent) is a cultivar that is similar to the recurrent parent with the additional trait from the donor parent. This methods is more efficient if the interest is to improve a current popular cultivar which has an obvious deficiency. Molecular markers have improved the efficiency and reduced the time to develop cultivars through backcrossing.
Visual Depiction of Types of Crosses
Material Transfer Agreement (MTA)
Legal Considerations
Seed or plant part material transfer agreement (MTA) is an agreement that allows for transfer of seed or plant part without transfer of title. The agreement is between the provider (one who provides seed) and the recipient (one who receives seed). Provider maintains ownership of the seed transferred at all times during the agreement dates and beyond. Transferred seed is received and used by the recipient according to the terms listed in the legally binding contract. In the agreement, the provider may impose conditions of audit or term bound reports on the usage of material and the recipient has to bind to these conditions. When a plant breeder makes request for seed, she/he will work with their organizational designate in the office of intellectual Property and Commercialization or the office that manages Intellectual Property (IP) and Technology Transfer. Similarly, the plant breeder should discuss with the same office when she/he receives a request for seed/plant part and should NEVER give out material without going through proper steps. MTA is signed by the sending breeder and his/her organizational representative, as well as recipient breeder and his/her organizational representative. Plant breeder or researcher utilizing the material (i.e., seed/plant part) is ultimately responsible for fulfilling the obligations of the MTA and therefore has to follow the regulations. Remember: MTA is a legal document.
MTA Sections
- Introduction: short text on the type of material or purpose
- Parties: describes the sender and recipient and their organizational affiliations
- Definitions: describes scientific terms such as material (seed and genotype etc.)
- Description of use of the materials: conditions on what can and cannot be done using the material
- Confidential information: lists any specific confidentiality clauses
- Intellectual Property rights: this is where licensing, royalties, inventions conditions are listed
- Warranties: This is to protect sender or provider that stipulates that material does not come with any warranties.
- Liability and/or indemnification: Recipient assumes all liability for damages that may arise from how/what recipient does to the material after transfer and sender is not liable.
- Publication: provides description on publication rights for receiver.
- Governing law: describes which jurisdiction laws will apply (state, country). In most cases where provider and recipient are from separate countries, the two parties may not define this section.
- Termination: date of termination of the agreement. It may also describe what the recipient has to do with leftover material from the agreement. Most likely original material sent by provider is expected to be destroyed.
- Signatures: Agreement is not considered executed until all necessary signatures are obtained and material should not be sent until MTA is signed and official. Signatories are the official organization representatives, provider breeder and recipient breeder. [note: provider and recipient may or may not be a breeder. For example, recipient or provider may be a geneticist]
- Exhibits or appendices: list of material, or data accompanying the material.
International Treaty on Plant Genetic Resources for Food and Agriculture
SMTA
The Standard Material Transfer Agreement (SMTA) is a mandatory model for parties wishing to provide and receive material under the Multilateral System. It is the result of lengthy negotiations among the Contracting Parties to the Treaty and may not be varied or abbreviated in any way. However, as a template, it contains some paragraphs and sections that need to be completed for each use.
The material transfer agreements that use the standard template are private agreements between the particular providers and recipients, but the Governing Body, through FAO as the Third Party Beneficiary, is recognized as having an interest in the agreements. The standard template has been developed to ensure that the provisions of the Treaty regarding the transfer of PGRFA under the Multilateral System are enforceable on users.”
Farmers’ Rights
“Farmers’ Rights: In its Article 9, the International Treaty recognizes the enormous contribution that the local and indigenous communities and farmers of all regions of the world, particularly those in the centers of origin and crop diversity, have made and will continue to make for the conservation and development of plant genetic resources which constitute the basis of food and agriculture production throughout the world. It gives governments the responsibility for implementing Farmers’ Rights, and lists measures that could be taken to protect and promote these rights:
- The protection of traditional knowledge relevant to plant genetic resources for food and agriculture;
- The right to equitably participate in sharing benefits arising from the utilization of plant genetic resources for food and agriculture; and
- The right to participate in making decisions, at the national level, on matters related to the conservation and sustainable use of plant genetic resources for food and agriculture.
Importance
The International Treaty also recognizes the importance of supporting the efforts of farmers and local and indigenous communities in the conservation and sustainable use of plant genetic resources for food and agriculture, including through a funding strategy. In this strategy, priority will be given to the implementation of agreed plans and programs for farmers in developing countries, especially in the least developed countries, and in countries with economies in transition, who conserve and sustainably utilize plant genetic resources for food and agriculture.”
It is important that breeders realize that if an MTA was signed to send or receive seed, it is a legal document and they are legally bound to follow the conditions.
Uses of germplasm: Methods to use germplasm in breeding programs include direct release as cultivars [less likely in species with breeding efforts, more likely in orphan crops where some selection may be done on a plant introduction or landrace prior to release as a cultivar]. The second and more appropriate use of germplasm is for introgression of single-gene traits from the wild species or unadapted germplasm into the elite cultivars.
References
Acquaah, G. 2012. Principles of Plant Genetics and Breeding, 2nd Edition. Wiley-Blackwell.
Brar, D.S., and G.S. Khush. 1997. Alien introgression in rice. Plant Molecular Biology 35: 35–47.
Harlan, J.R., and J.M.J. de Wet. 1971. Toward a Rational Classification of Cultivated Plants. Taxon, 20(4): 509-517.
Simmonds, N. 1979. Principles of Crop Improvement. Harlow, UK. Longman.
Singh, R.J., and R.L. Nelson. 2014. Methodology for creating alloplasmic soybean lines by using Glycine tomentella as a maternal parent. Plant Breeding. 133(5): 624-631.
Translocation is a type of chromosomal abnormality in which a chromosome breaks and a portion of it reattaches to a different chromosome.

