Chapter 9: Cowpea Breeding
Arti Singh; Teshale Mamo; Asheesh Singh; Anthony A. Mahama; and Walter Suza
Cowpea (Vigna unguiculata L. Walp.) (2n=2x=22) belongs to the Leguminosae family. Cowpea is an important legume crop ranked second after groundnut. It is grown for food and feed in multiple continents (Africa, Asia, Europe, the United States, and Central and South America). The center of origin and domestication is Southern Africa from where is was later carried to East and West Africa and Asia. Wild relatives of cowpea are found all over Africa. With grain comprised of 25% protein and several minerals and vitamins, it is another important crop that is vital for tackling current global food security challenges facing the world.
- Become familiar with the Cowpea crop
- List breeding institutions working on it
- Know classification system
- Describe adaptation and usage
- Outline production constraints
- Discuss breeding method used to develop pureline cowpea cultivars
- Outline a step by step breeding procedure using CB-27 cowpea cultivar as an example
Domestication and Diversification
Cowpea was domesticated in southern Africa and later spread to East and West Africa and Asia. Baudoin and Marechal (1985) classified domesticated cowpea into five cultivar groups (cultigroups).
- Unguiculata (seed testa thick and shiny) is the major group.
- Textilis (long inflorescence peduncle) is mostly found in West Africa.
- Sesquipedalis (fleshy pod, wrinkled when ripe) is mainly found in East Africa.
- Melanophthalmus (seed testa thin & often wrinkled, flower & seed partly white) originated in West Africa.
- Biflora (seed testa thick and shiny, flower and seed most often colored) is grown in South East Asia.
Biology of the Crop
General Characteristics and Development of the Crop

Cowpea is a warm-season, annual, herbaceous and similar in appearance to common bean (Phaseolus vulgaris L.) except that the leaves are generally darker green, shinier, and rarely pubescent. It has twining stems varying in erectness and bushiness. The trifoliate leaves develop alternatively, and petioles are 2 to 12 cm long. A wider range exists for leaf shape and size in cowpea than in common bean.
Plant growth habit is categorized as erect to semi-erect, prostrate (trailing type) or climbing, and indeterminate to determinate, depending on the genotype. However, most cowpea accessions have the indeterminate type of growth habit. Cowpea has a strong taproot system and the depth of the root has been measured up to 95 inches after 8 weeks of seeding. Flowers are born in axillary racemes on stalks with 15 to 30 cm peduncles. Usually, a single peduncle has two to three pods, however, under favorable growing conditions, a single peduncle often carries four or more pods. The presence of long peduncles is a unique feature of cowpea among legumes, and this characteristic facilitates hand harvesting. The cowpea flowers vary in color from white, cream and yellow to purple, and the seeds, which are smooth or wrinkled, range in color from white, cream or yellow to red, and are characterized by a marked hilum surrounded by a dark arc (Fig. 1).
Photosynthesis, Photoperiod, and Temperature
Cowpea is a short-day plant and like other grain legumes, cowpea processes its food using a C3 photosynthetic pathway. Different cowpea genotypes show photoperiod sensitivity in connection with floral bud initiation and development. Some genotypes are day-neutral, while other genotypes display a wider range of photoperiods (Craufurd et al. 1997). In addition, few cowpea genotypes exhibit various degrees of sensitivity to photoperiod (extent of delay in flowering) and temperature (Ehlers and Hall 1996). Warmer temperatures speed up flowering time in both photoperiod sensitive and insensitive cowpea genotypes. The development of improved cowpea genotypes for warm environments requires an understanding of the developmental responses to heat and photoperiod. Cowpea cultivars show a wide range of reproductive characteristics. The flower initiation ranges from 30 to 90 days after planting, and attaining physiological maturity (dry seed maturity) ranges from 55 to 240 days after planting (Wien and Summerfield, 1984). Wien and Summerfield (1984) reported that cowpea cultivars that flower early have a shorter or more concentrated flowering period than cultivars that flower late. In Sub-Saharan Africa, selection for different degrees of photosensitivity has occurred in different climatic zones and this resulted in pod ripening coinciding with the rainy season in some given locations. This condition helps the plant during pod set and ripening to escape damage from excessive rainfall and diseases attack. Therefore, photoperiod and temperature responses of particular cowpea genotypes allow cowpea breeders to make parental choices to best utilize exotic and adapted germplasm to serve particular environments.
General Classification
Classification by Utilization or Mode of Consumption
Cowpea is used as food as well as feed, including forage, hay and silage for livestock in Sub-Saharan Africa, Asia, Europe, USA and Central and South America. In Africa, people consume young leaves, immature pods, immature seeds and dried seeds. The stems, leaves, and vines of the cowpea serve as animal feed. Cowpea is also used as green manure and cover crop for maintaining the productivity of the soil. The grain contains 25% protein and several vitamins, minerals and fibers. Breeding efforts at the International Institute of Tropical Agriculture (IITA) and national programs have resulted in dual-purpose varieties (with good grain and fodder yields). The dual-purpose varieties have provided both grain and fodder while fitting the different cropping systems, economic, and climatic conditions encountered in Africa. In addition, cowpea has great flexibility in terms of its use as farmers can choose to harvest the cowpea for grains or for forage to feed their livestock, depending on economic or climatic conditions.
Classification by Seed Characteristics
Cowpea seed size ranges from small wild types to 0.5-1 cm long. The 1000 seed weight of cowpea is 150-300 grams. Most of the time, seeds develop a kidney shape if not restricted within the pod. If the development of seed is restricted by the pod, the seed becomes more globular. The seed coat in cowpea can either be smooth or wrinkled and an assortment of colors has been observed (including white, cream, green, buff, red, brown and black). Sometimes, the seed is either speckled or mottled. Many of the cowpea seeds are also referred to as eye bean (black eye, pinkeye purple hull) (Fig 2) where they are covered with a white tissue, with a blackish rim-like aril. In cowpea, the seed size is important because it directly influences productivity, and together with different color standards, can determine grain quality for the market. Therefore, seed size and color should also be considered as major traits of interest for breeding programs.

In the United States, different cowpea cultivar classes with a broad range in characteristics are grown for horticultural use. All cultivars that are grown in USA are day neutral members of the subspecies Unguiculata cultivar group Unguiculata. The cultivars grown for seed are classified as Blackeye beans, are known for good yield production), the Crowders type are known for their largest peas, and are often used for canning. Cream peas are the most popular and have become increasingly important for home gardening, while field types have few popular cultivars and most cultivars are old agronomic types.
Classification by Growth Habit
Cowpea has substantial genetic diversity for growth habit. The major growth habits are categorized as erect to semi-erect, prostrate (trailing) or climbing types. Growth habit in cowpea ranges from indeterminate to fairly determinate with the non-vining types tending to be more determinate. Meanwhile, some of the early maturing groups have a determinate growth types.
Classification by duration of Growth Period
Cowpea is grouped into early, medium and late maturity group. However, the range for growth-period duration varies from one region to another or among varieties of different growth habits. According to growth habit and region, cowpea cultivars range from 55 to 240 days to physiologically mature. The difference is not only varietal but also environmental, especially for the factors of day-length and temperature.
Adaptation and Economic Importance and Uses
Adaption
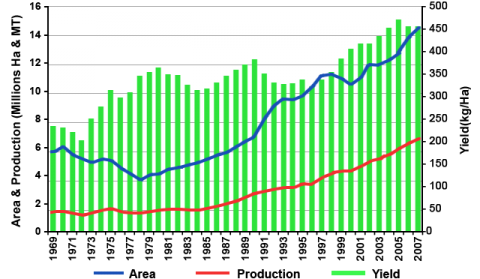
Cowpea is widely cultivated throughout the tropics and subtropics between 35°N and 30°S, across Africa, Asia and Oceania, the Middle East, Southern Europe, Southern USA and Central and South America. Cowpea is a crop adapted to hot and dry tropical conditions. It is also considered drought tolerant compared to other legumes. They grow best at low altitude with a precipitation of 400 to 700 mm per annum. Optimum crop production requires temperatures between 20-35°C during the growing season, and soil pH between 5.5 and 8.3. Cowpea is grown on a wide range of soil textures but the crop shows preference to sandy soil. It has low tolerance to salt but somewhat tolerant to aluminium. Like other legumes, the crop does not withstand waterlogged or flooded conditions. Cowpea is sensitive to chilling conditions. The crop is grown in 45 countries across the globe. An estimated 14 million ha is planted to cowpea each year across the globe with total annual production of about 6 million MT, the current average is estimated at about 0.45 tonnes/ha (FAOSTAT, 2010). The production trend of cowpea across the world is shown in a Fig. 3. Cowpea is primarily an African crop. The largest producers are Nigeria, Niger, Brazil, Haiti, India, Myanmar, Sri Lanka, Australia and the United States. Among these high cowpea producing countries, Nigeria and Niger each grow over 4 million ha and account for 45% and 15%, respectively, of the total world production (FAOSTAT, 2010).
Cowpea in the Human Diet and Nutrition
Cowpea is one of the most widely used legumes in the tropical parts of the world. It can be used at all growth stages as a vegetable crop. The grain is mainly used for human nutrition, making cowpea one of the most important dual purpose legumes. The nutritional content of cowpea grain is comparable to common beans, with relative low fat content. The protein in cowpea grains is rich in tryptophan compared to cereal grains. In Africa, immature green pods are used similar to snap bean in common bean.
Cropping System
Cowpea grows well in association with cereal crops through intercropping. Cowpea is a major component of the traditional cropping system in Africa, Asia, and Central and South America, where it is mainly grown with other crops in various combinations. It is grown as a millet-cowpea mixture (exhibit 22% of the field sampled), a predominant crop mixture system in the Sudan savanna of Nigeria (Henriet et al., 1997). In the dry savanna cropping system, millets have been grown with different crop mixtures including millet-sorghum-cowpea (represent 19%), sorghum-cowpea (10%) and millet-cowpea-groundnut (8 %) (Olufajo and Singh, 2002). Cowpea grain yield in the mixture is lower than under sole crop condition. The factors contributing to low yields under intercropping systems include low plant population, shading effects, and competition for nutrients. Cowpea is also used as green manure, where it is incorporated into soil and can provide nitrogen to subsequent crops, minimize soil erosion and suppresses weeds.
Production Constraints
Biotic Constraints
Several biotic factors that cause yield reduction in cowpea include insect pests, fungal, bacterial, viral diseases, plant parasites, other organisms.
- Insect Pests – Aphids are the main insect pests of cowpea, and are important vectors of cowpea mosaic virus. Other insect pests attacking cowpea are flower thrips and pod borers.
- Diseases – Cowpea diseases are due to fungi, bacteria and viruses. Examples of diseases include, Cercospora leaf spot, ashy stem blight, bacterial blight, blackeye cowpea mosaic polyvirus (BICMV), and cowpea mosaic comovirus.
- Plant Parasites – Certain weeds are important in cowpea production and most notable examples are the parasitic weedy plants Striga and Alectra.
- Nematodes – Nematode also causes root damage to the crop and result in significant yield loss.
Abiotic Constraints
Extreme drought and heat, soil acidity, low phosphorous are some of the abiotic factors that limit the yield of cowpea.
International Breeding Centers
The International Institute of Tropical Agriculture (IITA) has a global mandate for the development and improvement of cowpea. Its main duty and responsibility is to develop and distribute improved cowpea varieties to over 65 national cowpea research programs in Africa. Variety requirements for cowpea differ from region to region in respect of the seed color preference, use patterns, maturity and growth habit. Therefore, IITA located additional scientists and breeding centers in Philippines, Nigeria, Burkina Faso, Cameroon, Congo and Brazil in order to address the regional constraints in cowpea production at the global level.
A general strategy for IITA is to develop different cowpea breeding lines with diverse maturity (to feed specific adaptation across wide agro-ecological zones where cowpea is grown), plant type, and seed types combined with resistance to major biotic (diseases, insect-pests, and weeds) and abiotic (drought, heat and low phosphorous) stresses.
IITA’s genetic resources account for the world’s largest and most diverse pool of cowpea germplasm. The collection consists of over 15,000 cultivated varieties from over 100 countries, and 560 accessions of wild cowpeas (Singh et al., 1997). The IITA collection constitutes a valuable resource for the cowpea improvement worldwide. Scientists from IITA center and regional centers have identified various cowpea genotypes with numerous desirable genes, which govern plant architecture and physiological traits (like plant type, root architecture, growth habit, pod traits, seed traits, photosensitivity, maturity and nitrogen fixation), quality traits (fodder quality and grain quality), abiotic stress (heat and drought tolerances), biotic stress (resistance to major bacterial, fungal and viral diseases, resistance to rootknot nematodes, resistance to aphids, bruchid, thrips, and resistance to parasitic weeds such as Striga gesneriodes, and Alectra vogelii).
Breeding Methods and Strategies
Introduction
Cowpea is a true diploid species with a chromosome number of 2n = 2x = 22. It is primarily a self-pollinating crop in most production environments, although up to 5% outcrossing can occur in some environments, possibly associated with pollen transfer by insects. Different cowpea breeding programs have their own priority of target production zones including the cropping systems, consumption preferences and major constraints to cowpea production in their agro-ecological zones.
Most Cowpea breeders at IITA and National programs use bulk, backcross, and pedigree breeding methods to deal with large numbers of segregating populations because cowpea is an autogamous crop and most cultivars grown by farmers are pure lines. The primary objective in all cowpea breeding programs is higher grain yield and improved grain quality. In addition, to yield and quality traits, most breeders seek to breed in a wide range of abiotic and biotic stress resistance traits. The breeding strategy of IITA and regional breeding program is to develop broad range of breeding lines with high yield and adapted to different agro-ecological zones that possess regionally preferred characters for plant type, growth habit, days to maturity, seed type, combined with resistance to biotic and abiotic stress, along with quality. In general, the main focus of breeding programs is to develop extra early maturity (60-70 days) and medium maturity (75-90 days), non-photosensitive lines with good grain quality and possibility for dual purpose use, either for use as sole crop or as intercrop in multiple cropping systems.
Example of Cultivar Development
Development of Blackeye Cowpea Cultivar “CB27” at University of California Riverside
California Blackeye 27 (CB27) was developed by the University of California, Riverside (UCR) following the protocol shown in Fig. 4, and released in 1999 for its better performance in the following characteristics:
- High yielding
- Reproductive-stage heat tolerance
- Broad-based resistance to Fusarium wilt
- Broad-based resistance to root-knot nematodes
- Semi-dwarf and less vegetative shoot biomass
- Bright white seed coat
- Good seed weight
- Non-leaky pigments during boiling and excellent canning quality.
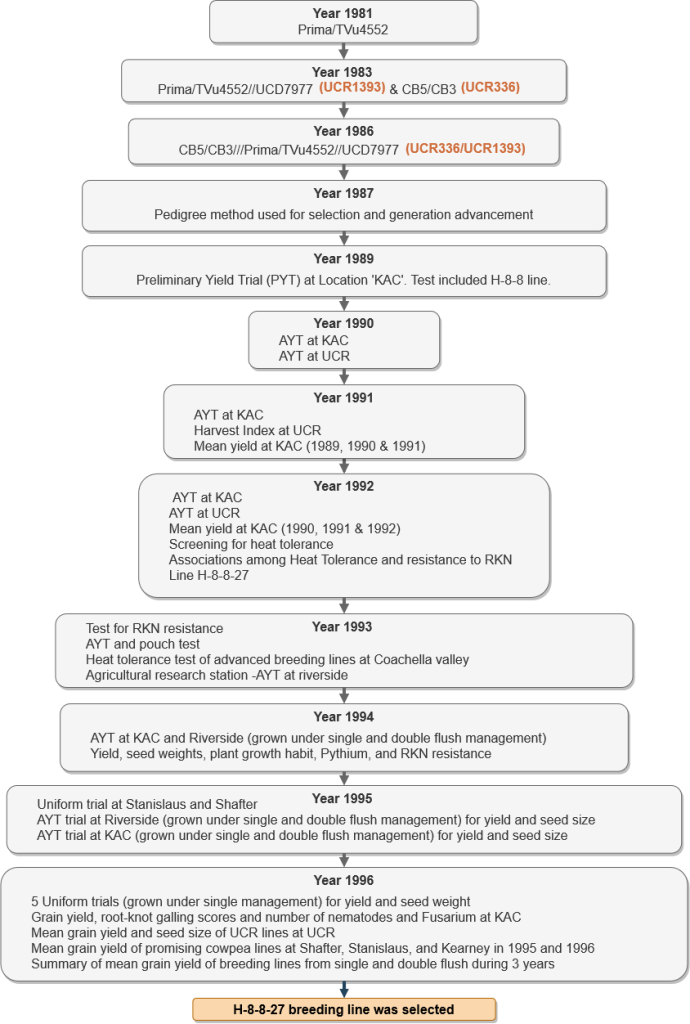
Actual data from Preliminary Yield Trials (PYT), Advanced Yield Trials (AYT) and Uniform Yield Trials (UYT) along with different test conducted on agronomic, disease and quality traits (from 1989 – 1998) led to the development of CB-27. Tables 1 – 20 show the results of various trials and years in which they were conducted to eventually release CB-27.
Note: all cells in Tables 1-20 with “n/a” are blank cells.
Year – 1989
| Entry | Origin | Score | Yield (lbs/acre) | Seed weight g/100 |
|---|---|---|---|---|
| H8-14 | 336 x 1393 | H | 3281 | 24.5 |
| H8-9 | 336 x 1393 | H | 3236 | 24.7 |
| H8-8 | 336 x 1393 | n/a | 3152 | 23.5 |
| H8-7 | 336 x 1393 | H | 3022 | 25.9 |
| H8-4 | 336 x 1393 | H | 2861 | 23.6 |
| CB5 | n/a | n/a | 2995 | 26.3 |
| CB46 | n/a | n/a | 3017 | 21.5 |
| LSD | n/a | n/a | 715 | 13 |
| CV (%) | n/a | n/a | 16.2 | 3.4 |
Year – 1990
| Entry | Origin | Yield (lbs/acre) | Seed weight (mg) | Seed density g/cm3 | Lodging | Earliness | Vigor |
|---|---|---|---|---|---|---|---|
| H8-14 | 336 x 1393 | 1805 | 235 | 1.10 | erect | early | compact |
| H8-9 | 336 x 1393 | 1805 | 248 | 1.09 | erect | early | compact |
| H8-8 | 336 x 1393 | 1497 | 233 | 1.11 | erect | early | compact |
| CB5 | CB x Iron | 1889 | 254 | 1.06 | erect | med | moderate |
| CB46 | CB5 x 166146 | 2274 | 224 | 1.09 | erect | med | moderate |
| LSD.05 | n/a | 266 | 10 | 0.03 | n/a | n/a | n/a |
| CV (%) | n/a> | 10 | 3 | 2 | n/a | n/a | n/a |
| Entry | Origin | Yield (lbs/acre) | Seed weight (mg) | Seed density g/cm3 | Lodging | Earliness | Vigor |
|---|---|---|---|---|---|---|---|
| H8-14 | 336 x 1393 | 2855 | 208 | 1.11 | erect | med | moderate |
| H8-9 | 336 x 1393 | 3007 | 223 | 1.14 | slight | med | moderate |
| H8-8 | 336 x 1393 | 2744 | 217 | 1.12 | erect | early | compact |
| CB5 | CB x Iron | 2389 | 241 | 1.11 | erect | early | compact |
| CB46 | CB5 x 166146 | 2688 | 202 | 1.17 | erect | med | moderate |
| LSD.05 | n/a | 420 | 9 | 0.02 | n/a | n/a | n/a |
| CV (%) | n/a | 11 | 3 | 1 | n/a | n/a | n/a |
Year – 1991
| Entry | Origin | Yield (lbs/acre) | Seed weight (mg/seed) | Seed density g/cm3 |
|---|---|---|---|---|
| H8-14 | 336 x 1393 | 2825 | 195 | 1.10 |
| H8-9 | 336 x 1393 | 3647 | 227 | 1.09 |
| H8-8 | 336 x 1393 | 306 | 217 | 1.10 |
| CB5 | CB x Iron | 2244 | 234 | 1.09 |
| CB46 | CB5 x 166146 | 2514 | 200 | 1.15 |
| LSD.05 | n/a | 374 | 15 | 0.01 |
| CV (%) | n/a | 10 | 5 | 1 |
| A. Entry | Mean Grain Yield (lbs/acre) 1990 & 1991 | Seed weight (mg) |
|---|---|---|
| H8-14 | 2840 | 202 |
| H8-9 | 3327 | 225 |
| H8-8 | 2902 | 217 |
| CB5 | 2316 | 238 |
| CB46 | 2601 | 201 |
| CB88 | 2662 | 221 |
| B. Entry | Mean Grain Yield (lbs/acre) 1989-1991 | Seed weight (mg) |
| H8-14 | 2987 | 216 |
| H8-9 | 3297 | 232 |
| H8-8 | 2985 | 223 |
| CB5 | 2543 | 246 |
| CB46 | 2740 | 206 |
| Entry | Harvest Index % | Seed weight (mg/seed) |
|---|---|---|
| H8-14 | 43 | 247 |
| H8-9 | 51 | 232 |
| H8-8 | 43 | 234 |
| CB5 | 43 | 252 |
| CB46 | 47 | 203 |
| LSD.05 | 4 | 16 |
| CV (%) | 7 | 5 |
Year – 1992
| Entry | Origin | Yield (lbs/acre) | Seed weight (mg/seed) | Seed density g/cm3 |
|---|---|---|---|---|
| H8-14 | 336 x 1393 | 2949 | 213 | 1.01 |
| H8-9 | 336 x 1393 | 2805 | 219 | 1.02 |
| H8-8 | 336 x 1393 | 2865 | 209 | 1.02 |
| CB5 | CB x Iron | 2831 | 229 | 1.00 |
| CB46 | CB5 x 166146 | 3164 | 194 | 1.06 |
| LSD.05 | n/a | 404 | 11 | 0.02 |
| CV (%) | n/a | 10 | 3.5 | 1.3 |
| Entry | Origin | Yield (lbs/acre) | Seed weight (mg/seed) | Seed density g/cm3 |
|---|---|---|---|---|
| H8-14 | 336 x 1393 | 2777 | 209 | 0.98 |
| H8-9 | 336 x 1393 | 2684 | 207 | 0.97 |
| H8-8 | 336 x 1393 | 2615 | 216 | 0.97 |
| CB5 | CB x Iron | 2373 | 225 | 0.95 |
| CB46 | CB5 x 166146 | 2461 | 193 | 0.99 |
| LSD.05 | n/a | 318 | 19 | 0.03 |
| CV (%) | n/a | 85 | 6.0 | 2.0 |
| Entry | Yield (MEAN of years 1990, 1991 and 1992) | Seed weight (mg) |
|---|---|---|
| H8-14 | 2876 | 206 |
| H8-9 | 3153 | 223 |
| H8-8 | 2890 | 214 |
| CB5 | 2488 | 235 |
| CB46 | 2789 | 199 |
| Line | Total # of Sub-lines |
# Heat Tolerance – Flowering (CVARS & GH) |
Heat Tolerance – % Podding (Hot Glasshouse at UCR) |
# Selected sublines |
Average Podding |
|---|---|---|---|---|---|
| H8-14 | 45 | 26 | 57 | 9 | 4.7 |
| H8-9 | 54 | 54 | 92 | 14 | 9.4 |
| H8-8 | 46 | 24 | 70 | 12 | 5.0 |
| Line | 1Nematode Resistance | #2Heat Tolerance – Floral buds | #2Heat Tolerance – Pod set |
|---|---|---|---|
| H8-8-1 | R | N | 3 |
| H8-8-2 | S | S | – |
| H8-8-3 | R | N | 6 |
| H8-8-4 | S | S | – |
| H8-8-5 | R | N | 2 |
| H8-8-6 | S | S | – |
| H8-8-8 | R | N | 0 |
| H8-8-9 | R | N | 7 |
| H8-8-10 | R | N | 0 |
| -to- | n/a | n/a | n/a |
| —- | n/a | n/a | n/a |
| H-8-8-27 | R | N | n/a |
Year – 1993
The blackeye cowpea cultivators follow three management schemes:
- Single-flush main crop cut after ~ 100 days
- Single-flush double crop, sown later and cut after ~ 100 days
- Double-flush main crop, sown early and cut after ~ 140 days
Short-term goal – to develop blackeye varieties with resistance to the:
- common race of Fusarium wilt in California (race #3)
- wide range of root knot nematodes
- heat tolerance
- increased yield potential
Medium-term goal – to develop blackeye varieties that have resistance to early cut-out and greater ability to produce pods over an extended season (140 days from planting to cutting)
Long-term goal – resistance to lygus, resistance to cowpea aphid
| Line | Date of Test | |||
| 13-May | 27-Feb | 25-Aug | R/S | |
| CB5 | 2 | n/a | n/a | R |
| CB46 | 2 | n/a | n/a | R |
| H-8-8-2 | 2 | 0 | n/a | R |
| H-8-8-4 | 4 | <1 | n/a | R |
| H-8-8-6 | <1 | <1 | 0 | R |
| H-8-8-8 | 2 | 0 | n/a | R |
| H-8-8-13 | 0 | 0 | 0 | R |
| H-8-8-15 | 0 | 0 | 0 | R |
| H-8-8-16 | 10 | 0 | 0 | R |
| H-8-8-27 | <1 | <1 | <1 | R |
| H-8-8-28 | 5 | n/a | n/a | R |
| H-8-8-30 | 0 | n/a | n/a | R |
| H-8-8-32 | 43 | n/a | n/a | S |
| H-8-8-35 | 0 | <1 | <1 | R |
| Line | Date of Test | Classification | ||
| May 13 | April 9 | Aggressive | Non-aggressive | |
| CB3 | 32 | 190 | S | S |
| CB46 | 15 | 24 | S | R |
| H-8-8-2 | 6 | n/a | R | R |
| H-8-8-4 | n/a | 4 | R | R |
| H-8-8-6 | 7 | 12 | R | R |
| H-8-8-8 | n/a | 17 | R | R |
| H-8-8-13 | 2 | 29 | R | R |
| H-8-8-15 | 6 | 7 | R | R |
| H-8-8-16 | 11 | 20 | R | R |
| H-8-8-27 | 2 | 31 | R | R |
| H-8-8-31 | n/a | 85 | S | S |
| H-8-8-35 | 8 | 22 | S | R |
| Entry | Grain Yield g/plant | Plots/Plant | Seeds/Pod | Seed Weight Mg/seed | Flower Production | Pods/Peduncle # |
|---|---|---|---|---|---|---|
| CB5 | 0 | 0 | n/a | n/a | NO | n/a |
| CB46 | 2 | 4 | 2.7 | 166 | NO | n/a |
| H8-8-6 | 22 | 28 | 4.2 | 192 | YES | 2.5 |
| H-8-8-13 | 21 | 27 | 4.1 | 190 | YES | 2.75 |
| H-8-8-15 | 22 | 27 | 4.1 | 196 | YES | 3.00 |
| H-8-8-16 | 30 | 34 | 4.6 | 195 | YES | 2.75 |
| H-8-8-27 | 26 | 30 | 4.2 | 207 | YES | 2.75 |
| H-8-8-35 | 28 | 30 | 4.6 | 201 | YES | 2.75 |
| Entry | Grain Yield lbs/ac | Seed weight mg/seed | Heat tolerance | Root Knot Resistance | |
| Non-aggressive | Aggressive | ||||
| CB5 | 1975 | 260 | SUS | RES | SUS |
| CB46 | 1996 | 225 | SUS | RES | SUS |
| H8-8-6 | 1631 | 240 | TOL | RES | RES |
| H-8-8-13 | 1951 | 228 | TOL | RES | RES |
| H-8-8-15 | 1938 | 227 | TOL | RES | RES |
| H-8-8-16 | 2156 | 231 | TOL | RES | RES |
| H-8-8-27 | 1767 | 246 | TOL | RES | RES |
| H-8-8-35 | 2049 | 229 | TOL | RES | RES |
| LSD.05 | 405 | 22 | n/a | ||
| CV% | 15 | 6 | |||
Year – 1994
| Entry | Riverside Single Flush | Riverside Double Flush | Kearney Single Flush | Kearney Double Flush | Mean |
|---|---|---|---|---|---|
| CB-46 | 1860 | 2996 | 3046a | 3916 | 2955 |
| CB-5 | 2063 | 2869 | 2222 | 3479 | 2658 |
| H8-8-1N | 1985 | 3199 | 2629 | 3786 | 2900 |
| H8-8-6 | 2039 | 2677 | 2051 | 3366 | 2531 |
| H8-8-13 | 1800 | 2318 | 2637 | 2717 | 2668 |
| H8-8-15 | 1703 | 3040 | 2589 | 3175 | 2627 |
| H8-8-27 | 1742 | 2979 | 2398 | 3728 | 2712 |
| H8-8-35 | 1771 | 2658 | 2414 | 3399 | 2561 |
| LSD(.05) | NS | 581 | 245 | NS | 288 |
| CV(%) | 13 | 14 | 15 | 16 | 16 |
| Entry | Riverside Single Flush | Riverside Double Flush | Kearney Single Flush | Kearney Double Flush | Mean |
|---|---|---|---|---|---|
| CB-46 | 234 | 211 | 221 | 211 | 219 |
| CB-5 | 273 | 255 | 265 | 246 | 260 |
| H8-8-1N | 249 | 233 | 242 | 225 | 237 |
| H8-8-6 | 243 | 238 | 230 | 220 | 233 |
| H8-8-13 | 231 | 206 | 209 | 213 | 215 |
| H8-8-15 | 239 | 227 | 240 | 218 | 231 |
| H8-8-27 | 240 | 229 | 242 | 221 | 233 |
| H8-8-35 | 237 | 220 | 234 | 222 | 228 |
| LSD(.05) | 11 | 12 | 6 | 13 | 6 |
| CV(%) | 3.1 | 3.6 | 3.9 | 3.9 | 3.6 |
| Entry | Growth habit Plant Size | Growth habit vinyness | Pythium |
|---|---|---|---|
| CB-46 | M | M-L | 5.3 |
| CB-5 | L | H | 7.5 |
| H8-8-1N | M-L | H | 2.3 |
| H8-8-6 | L | M | 2.5 |
| H8-8-13 | M | M | 4.3 |
| H8-8-15 | M | L | 1.3 |
| H8-8-27 | M | L | 1.5 |
| H8-8-35 | M | L | 1.3 |
| LSD(.05) | n/a | n/a | 4.3 |
| Entry | Grain yield lbs/ac | Seed weight mg/seed | RKN resistance non-aggr | RKN resistance aggr | RKN resistance javanica |
|---|---|---|---|---|---|
| CB-46 | 3481a | 216 | R | S | S |
| CB-5 | 2851 | 255 | R | S | S |
| H8-8-1N | 3208a | 234 | R | S | S |
| H8-8-6 | 2709 | 225 | R | R | R |
| H8-8-13 | 2677 | 211 | R | S | S |
| H8-8-15 | 2882 | 229 | R | R | R |
| H8-8-27 | 3063a | 231 | R | R | R |
| H8-8-35 | 2907 | 228 | R | R | R |
| H8-8-35 | 2907 | 228 | n/a | n/a | n/a |
| LSD(.05) | 472 | 9 | n/a | ||
| CV(%) | 16 | 3.9 | |||
Year – 1995
| Entry | Stanislaus | Shafter | Mean |
|---|---|---|---|
| H8-8-27 | 24.5 | 55.5 | 40.2 |
| H8-8-15 | 23.0 | 55.2 | 39.1 |
| CB46 | 20.8 | 55.9 | 38.4 |
| CB88 | 11.5 | 54.3 | 32.9 |
| LSD(.05) | 3.4 | 3.8 | 2.5 |
| CV(%) | 14.8 | 6.1 | 8.5 |
| Entry | Origin | Single Flush | Double Flush | Mean |
|---|---|---|---|---|
| H8-8-27 | CB5/CB3//1393 | 22.3 | 29.6 | 26.0 |
| H8-8-15 | CB5/CB3//1393 | 23.9 | 29.2 | 26.6 |
| CB46 | CB5/CB3//PI1166146 | 23.3 | 25.9 | 24.6 |
| CB88 | CB5/CB3//PI1166146 | 24.5 | 29.6 | 27.1 |
| LSD(.05) | 2.1 | NS | 3.4 | n/a |
| CV(%) | 8 | 14 | 16 |
| Entry | Origin | Single Flush | Double Flush | Mean |
|---|---|---|---|---|
| H8-8-27 | CB5/CB3//1393 | 38.3 | 42.8 | 41.3 |
| H8-8-15 | CB5/CB3//1393 | 38.3 | 41.9 | 40.1 |
| CB46 | CB5/CB3/PI1166146 | 31.7 | 47.2 | 39.4 |
| CB88 | CB5/CB3/PI1166146 | 34.0 | 44.4 | 39.2 |
| LSD(.05) | 4.6 | 8.0 | 4.8 | n/a |
| CV(%) | 12 | 16 | 15 |
| Line | Nematodes Non-aggres | Nematodes aggres | Nematodes M.jay. | Fusarium Race 3 | Fusarium Race 4 | Heat |
|---|---|---|---|---|---|---|
| H8-8-27 | Yes | Yes | Yes | Yes | Yes | Yes |
| H8-8-15 | Yes | Yes | Yes | Yes | Yes | Yes |
| CB46 | Yes | No | No | Yes | Yes | No |
| CB5 | Yes | No | No | No | Yes | No |
| Notes: Types of RKN; Non-aggres = Non-aggressive M. incognita – not able to overcome standard ‘Rk’ gene resistance. aggres = strain of M. incognita – able to overcome ‘Rk’ resistance |
||||||
| Line | Kearney | Riverside | Mean |
|---|---|---|---|
| H8-8-27 | 208 | 215 | 212 |
| H8-8-15 | 207 | 209 | 208 |
| CB46 | 203 | 201 | 202 |
| CB88 | 216 | 210 | 213 |
| LSD(.05) | 11 | 19 | 11 |
| CV(%) | 4.4 | 7.7 | 6.3 |
| Line | 1994 | 1995 | Mean |
|---|---|---|---|
| H8-8-27 | 231 | 212 | 222 |
| H8-8-15 | 229 | 208 | 219 |
| CB46 | 216 | 202 | 209 |
| CB88 | 231 | 213 | 222 |
| LSD(.05) | 6 | 11 | n/a |
| Line | Mean |
|---|---|
| H8-8-27 | 33 |
| H8-8-15 | 33 |
| CB46 | 33 |
| CB88 | 32 |
Year – 1996
| Entry | Shafter | Tulare | Kearney double | Kearney single | Westside | Mean | Seed weight mg/seed |
|---|---|---|---|---|---|---|---|
| H8-8-27 | 53.1 | 42.6 | 38.0 | 26.1 | 24.5 | 36.9 | 213 |
| H8-8-15 | 45.8 | 40.3 | 33.3 | 23.9 | 23.8 | 33.4 | 207 |
| CB46 | 46.6 | 49.1 | 40.5 | 25.5 | 24.6 | 37.3 | 215 |
| CB88 | 46.2 | 41.1 | 37.0 | 23.4 | 20.9 | 33.7 | 215 |
| LSD.05 | 8.3 | 8.1 | NS | 2.7 | 1.4 | 2.7 | 7 |
| CV(%) | 11.7 | 12.5 | 13.8 | 7.7 | 4.0 | 12.1 | 2.1 |
| Entry | Grain Yield (KAC) | Grain Yield (Muller) | Galling (KAC) | Galling (Muller) | No. juveniles (KAC) | No. juveniles (Muller) | Resistance Nematode | Fusarium Wilt |
|---|---|---|---|---|---|---|---|---|
| H8-8-27 | 21.1 | 21.0 | 2.3 | 2.1 | 1672 | 328 | Rk+ | 3 & 4 |
| H8-8-15 | 19.9 | 23.1 | 1.6 | 2.0 | 1061 | 239 | Rk+ | 3 & 4 |
| CB46 | 21.2 | 16.5 | 4.9 | 4.7 | 1833 | 572 | Rk | 3 |
| CB88 | 22.9 | 10.2 | 4.5 | 5.1 | 2478 | 572 | Rk | 3 |
| LSD.05 | 2.9 | 3.1 | 0.6 | 0.9 | 750 | NS | n/a | n/a |
| Entry | Grain Yield (Single) | Grain Yield (Double) | Seed Size (Single) | Seed Size (Double) | Means Yield | Means Seed Size |
|---|---|---|---|---|---|---|
| H8-8-27 | 20.8 | 32.7 | 223 | 249 | 26.8 | 236 |
| H8-8-15 | 22.4 | 36.1 | 226 | 245 | 29.3 | 236 |
| CB46 | 24.7 | 34.9 | 223 | 234 | 29.8 | 228 |
| CB88 | 22.7 | 34.7 | 226 | 232 | 28.7 | 229 |
| LSD.05 | 2.5 | NS | 9 | 9 | 2.5 | 6 |
| CV(%) | 7.5 | 9.0 | 2.7 | 2.5 | 8.7 | 2.6 |
| Entry | Shafter mean Yield 1995 and 1996 | Stanislaus mean Yield 1995 and 1996 | Kearney Mean Yield 1995 and 1996 | Overall Mean |
|---|---|---|---|---|
| H8-8-27 | 55 | 23 | 34 | 36 |
| H8-8-15 | 50 | 23 | 33 | 34 |
| CB46 | 51 | 19 | 36 | 36 |
| CB88 | 50 | 11 | 33 | 31 |
| Entry | 1994 Single flush | 1994 Double flush | 1995 Single flush | 1995 Double flush | 1996 Single flush | 1996 Double flush | Mean Single | Mean Double |
|---|---|---|---|---|---|---|---|---|
| H8-8-27 | 30.5 | 39.2 | 31.7 | 47.2 | 25.5 | 40.5 | 29.2 | 42.3 |
| H8-8-15 | 25.1 | 35.5 | 34.0 | 44.4 | 23.4 | 37.0 | 27.5 | 39.0 |
| CB46 | 25.9 | 31.8 | 38.3 | 42.8 | 23.9 | 33.3 | 29.4 | 36.0 |
| CB88 | 24.0 | 37.3 | 38.3 | 41.9 | 26.1 | 38.0 | 29.5 | 39.1 |
| LSD.05 | 2.4 | NS | 4.6 | 8.0 | 2.7 | NS | 2.2 | 4.2 |
| CV(%) | 15 | 16 | 12 | 16 | 8 | 14 | 10 | 13 |
Year – 1997
| Entry | Grain Yield (Dirt Wt) Cwt/ac |
Grain Yield (Clean Wt) Cwt/ac |
Clean Out % | Bean Size Gm/100 seeds |
Total Damage (%) |
Splits | Grade |
|---|---|---|---|---|---|---|---|
| H8-8-27 | 49 | 44 | 8.1 | 24.1 | 2.0 | 0.3 | UN No. 1 |
| CB46 | 48 | 44 | 7.7 | 21.7 | 4.2 | 0.3 | US No. 3 |
| Entry | Westside | Riverside | Tulare | Shafter | Mean |
|---|---|---|---|---|---|
| H8-8-27 | 1790 | 3491 | 2840 | 4446 | 3147 |
| CB46 | 1990 | 3772 | 3769 | 5231 | 3638 |
| LSD(.05) | NS | 472 | 302 | NS | 241 |
| CV(%) | 11.4 | 7.8 | 7.0 | 16.6 | 11.3 |
| Entry | Shafter Mean Yield 1995, 1996, and 1997 | Kearney mean Yield 1994, 1995, 1996, and 1997 | Overall Mean |
|---|---|---|---|
| H8-8-27 | 51 | 35 | 42 |
| H8-8-15 | 51 | 33 | 41 |
| Seed size | |||
| CB46 | n/a | n/a | 21.1 |
| CB88 | n/a | n/a | 22.1 |
| Entry | Riverside Yield | Riverside Greenness | Tulare Yield | Tulare Greenness | Mean Yield | Means Greenness |
|---|---|---|---|---|---|---|
| H8-8-27 | 3491 | 0 | 2840 | 0.7 | 3166 | 0.4 |
| CB46 | 3772 | 0 | 3769 | 2.2 | 3771 | 1.1 |
| LSD.05 | 472 | n/a | 699 | n/a | 302 | n/a |
| CV(%) | 7.8 | n/a | 7.0 | n/a | 7.6 | n/a |
1998 – Uniform Blackeye Trials
| Entry | Shafter | Tulare | Kearney | Riverside | Mean |
|---|---|---|---|---|---|
| H8-8-27 | 5156 | 4967 | 4629 | 3113 | 4466 |
| CB46 | 4732 | 5178 | 4268 | 2938 | 4271 |
| LSD(.05) | 521 | 478 | 806 | 529 | 295 |
| CV(%) | 8 | 6 | 13 | 13 | 10 |
| Entry | Shafter | Tulare | Kearney | Riverside | Mean | % Split |
|---|---|---|---|---|---|---|
| H8-8-27 | 22.8 | 4967 | 22.6 | 24.9 | 23.0 | 13 |
| CB46 | 22.6 | 5178 | 21.9 | 25.8 | 23.0 | 18 |
| LSD.05 | 1.3 | 478 | 1.1 | 1.7 | 0.7 | 6 |
| CV(%) | 4 | 6 | 3 | 5 | 4 | 42 |
| Genotype | Spacing | Yield | HI % | Seed weight g/100 seed | Seeds/pod | Pods/peduncle |
|---|---|---|---|---|---|---|
| Compact type | ||||||
| H8-8-27 | 30″ | 3642 | 47.0 | 24.7 | 8.4 | 1.7 |
| n/a | 40″ | 3407 | 50.6 | 24.6 | 8.7 | 1.5 |
| n/a | 40″ x 2 | 4111 | 47.1 | 23.8 | 8.4 | 1.5 |
| n/a | Mean | 3721 | 48.2 | 24.4 | 8.5 | 1.6 |
| CB46 | 30″ | 3583 | 47.0 | 23.6 | 8.5 | 2.1 |
| n/a | 40″ | 3081 | 45.8 | 24.1 | 8.3 | 1.9 |
| n/a | 40″ x 2 | 3692 | 42.7 | 24.2 | 8.6 | 1.6 |
| n/a | Mean | 3665 | 45.2 | 24.0 | 8.5 | 1.9 |
| Genotype | Spacing | Yield | HI % | Seed weight g/100 seed | Seeds/pod | Pods/peduncle |
|---|---|---|---|---|---|---|
| Compact type | ||||||
| H8-8-27 | 30″ | 2717 | 48 | 23.8 | 8.4 | 2.1 |
| n/a | 40″ | 2399 | 48.1 | 23.3 | 8.8 | 2.2 |
| n/a | 40″ x 2 | 2643 | 48.2 | 23.7 | 8.0 | 2.0 |
| n/a | Mean | 2587 | 48.1 | 23.6 | 8.4 | 2.1 |
| CB46 | 30″ | 2472 | 42.0 | 23 | 9.5 | 1.9 |
| n/a | 40″ | 2328 | 45.5 | 21.9 | 8.0 | 2.0 |
| n/a | 40″ x 2 | 2498 | 44.0 | 23.3 | 8.1 | 1.8 |
| n/a | Mean | 2432 | 43.8 | 22.8 | 8.5 | 1.9 |
Year – 1999
| Entry | Fusarium wilt Race 3 | Fusarium wilt Race 4 | RKN (M. incognita) Avirulent | RKN (M. incognita) Virulent | RKN (M. javanica) | Heat Tolerance | Chill Tolerance |
|---|---|---|---|---|---|---|---|
| CB5 | No | No | Yes | No | No | No | No |
| CB46 | Yes | No | Yes | No | No | No | No |
| CB27 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Example Using Participatory Varietal Selection
Recent example of Cowpea cultivar released by IITA in parts of Africa using participatory varietal selection.
- In Burkina Faso, two improved cowpea varieties developed by IITA have been released.
- IT99K-573-2-1 and
- IT98K-205-8,
- Using participatory varietal selection approach, local farmers and researchers choose varieties from various options after two years of trial in the central and northern regions of Burkina Faso.
- Selected varieties are early maturing (60 days), high yielding (2170 kg/ha), resistant to parasitic weed striga along with big size, preferred color, and cooking qualities pertaining to farmers’ taste.
- New cowpea varieties also have better adaptability to climate change and can be grown successfully in drier regions with low rainfall.
Important Traits
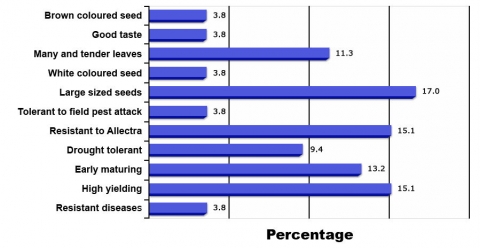
Example of Participatory market-led cowpea breeding in Sub-Saharan Africa (Tanzania and Malawi) in assigning importance to traits (Fig. 5)
Pathway Based on Preferences
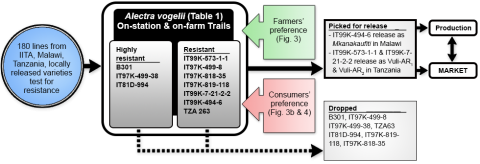
Farmers’ and consumers’ preferences of traits in a variety or cultivar play a critical role on the release and adoption of new varieties. It is important to note that the preferences of the two groups differ and therefore, require the close attention needed to address those preferences (Fig. 6).
Notes to Consider
- Alectra vogelii is a parasitic weed that causes considerable damage to cowpea plant by attaching to it and tapping nutrients.
- In Tanzania and Malawi, Alectra is one of the major weed growing in almost all cowpea growing areas.
- In Figure 4 is shown important traits of cowpea required by farmers. Out of 11 traits used in selection of best cowpea lines by farmers, only five traits (brown seed color, white seed color, good taste, large seed, many leaves and tender leaves) are specific to the final consumer, while the other six traits (early maturity, high yield, resistance to Alectra, resistance to diseases, tolerant to pest, drought tolerance) are agronomic traits. Large seed size is the most important trait from marketing perspective, whereas high yield, early maturity, and resistance to A. vogelli are the main agronomic traits which are the deciding criteria used by farmers to select varieties for growing on their farm.
- In Figure 5 is shown an example of value chain approach used to develop cultivars (for example-IT99K-573-2-1)
- This approach resolves biases and takes care of farmers, consumers and market preference and will not let breeders effort go waste like in past where outstanding varieties with excellent agronomic traits failed due to inability to satisfy needs of farmers, consumers and market at the same time.
Marker-Assisted Selection
Marker-assisted selection approaches are being developed in cowpea with high-density marker maps and SNP markers becoming available. As cowpea is gaining acreage globally more investment is being made for breeding and marker development. This will assist in further development of MAS in cowpea. Genetic loci controlling important pest and disease resistance genes and agronomic traits have been placed on the genetic map (for example, Kelly et al, 2003). Closely linked markers to some of the biotic traits have been identified (Gowda et al., 2002). Most of these traits are governed by major genes and are potentially good candidates for MAS. Along with MAS for simply inherited traits, the genomic selection approach offers usefulness in future breeding efforts. Currently, joint efforts are being made by IITA, Bean/Cowpea Collaborative Research Support Program (Bean/Cowpea CRSP), advanced laboratories in the USA, Australia, African Agricultural Technology Foundation (AATF), Network for Genetic Improvement of Cowpea for Africa (NGICA) and Monsanto Corporation to exploit biotechnology tools to complement conventional breeding methods for improving resistance to diseases and insects.
References
Abate T., A.D. Alene, D. Bergvinson, B. Shiferaw, S. Silim, A. Orr, and S. Asfaw. 2012. Tropical Grain Legumes in Africa and South Asia: Knowledge and Opportunities. PO Box 39063, Nairobi, Kenya: International Crops Research Institute for the Semi Arid Tropics. 112 pp. ISBN: 978-92-9066-544-1. Order Code: BOE 056.
Baudoin J.P., and R. Maréchal. 1985. Genetic diversity in Vigna. In: Singh SR, Rachie KO (eds) Cowpea research, production, and utilization. Wiley, New York, pp 3-11.
Ehlers J.D. and A.E. Hall. 1996. Genotypic Classification of Cowpea Based on Response to Heat and Photoperiod. Crop Science 36: 673-679.
Hella, J.P., T. Chilongo, A.M. Mbwag, J. Bokosi, V. Kabambe, C. Riches, and C.L. Massawe. 2013. Participatory market-led cowpea breeding in Sub-Saharan Africa: Evidence pathway from Malawi and Tanzania. Merit Research Journal of Agricultural Science and Soil Sciences (ISSN:2350-2274) Vol. 1(2) pp. 011-018.
Henriet, J., G.A. van EK, S.F. Blade, and B.B. Singh. 1997. Quantitative assessment of traditional cropping systems in the Sudan savanna of Northern Nigeria, I. Rapid survey of prevalent cropping systems. Samara Journal of Agricultural Research 14: 37-45.
Heuzé V., Tran G., 2015. Cowpea (Vigna unguiculata) seeds. Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO. https://www.feedipedia.org/node/232 Last updated on May 11, 2015, 14:31
Kelly, J.D., Gepts P, Miklas PN, Coyne DP. (2003). Tagging and mapping of genes and QTL and molecular marker-assisted selection for traits of economic importance in bean and cowpea. Field Crops Res. 82:135-154.
Olufajo, O.O., and B.B. Singh. 2002. Advances in cowpea cropping systems research. Pp 267-277 in challenges and opportunities for enhancing sustainable cowpea production. Proceedings of world cowpea conference III held at International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. 4-8 September 2000. IITA. Ibadan Nigeria.
Singh, B.B., O.L. Chamblis, and B. Sharma. 1997. Recent advances in cowpea breeding. Pages 3049 in Advances in Cowpea Research, edited by B.B. Singh, D.R. Mohan Raj, K.E. Dashiell, and L.E.N. Jackai. IITA, and Japan International Research Centre for Agricultural Sciences (JIRCAS) copublication. Available at IITA, Ibadan, Nigeria.
Timko, M.P., J.D. Ehlers, and P.A. Roberts: Cowpea. In Genome Mapping and Molecular Breeding in Plants, Pulses, Sugar and Tuber Crops. Volume 3. Edited by Kole C. Berlin: Springer-Verlag; 2007: 49-68.

