Chapter 4: New Line Development and New Line Evaluation: Clonally Propagated Cultivars
Rita H. Mumm

In Chapter 2, the process and concepts of New Line Development and Evaluation were examined using soybean as an example of a pure line variety of a self-pollinated crop. Then, in Chapter 3, this process was analyzed with respect to changes needed to accommodate hybrid cultivars such as single-cross hybrid maize.
In this chapter, we will examine the changes to the process and the product pipeline that are unique to developing improved clonally propagated cultivars. The example cited is the potato (the so-called Irish potato, Solanum tuberosum subsp. tuberosum) (Fig. 1).
What are Clonally Propagated Crops?
Clonally propagated crops are those maintained and distributed for cultivation by asexual reproduction. Across all clonally propagated crops, different plant parts may be used as clones, e.g. tubers, roots, and stem cuttings. Potato varieties are maintained as tubers (Fig. 2).

Features of Clonally Propagated Crops
Most clonally propagated crops are cross-pollinated; many are obligate outcrossers due to self-incompatibility. This means that typically parents used in breeding crosses are highly heterozygous, and heterozygous F1 individuals produced by crossing comprise the breeding population. Thus, the family structure in the breeding population begins with a single plant at the F1 stage. Actually, each F1 plant is a potential variety!
The high degree of heterozygosity facilitates exploitation of heterosis. The level of heterozygosity is amplified by the fact that many clonally propagated crops are polyploid. For example, potato can be 2x, 3x, 4x, or 5x; Irish potato is tetraploid. Theoretically, every allele at a locus could be distinctive.
All released varieties of clonally propagated crops are homogeneous: i.e. varieties are non-segregating, genetically fixed, and completely uniform. At the same time, each variety may be a highly heterozygous hybrid.
Exploiting Genetic Variation
New genetic variation is generated by sexual reproduction in crossing parent lines. Once the resulting seed has been produced, no genetic changes occur. All clones created from each F1 seed plant are genetically identical. Asexual reproduction is used to generate all subsequent generations of materials derived from the F1 seed plants.
Thus, all genetic variation can be exploited in clonally propagated crops, i.e., additive, dominance, and epistatic variation. The covariance between clones (of different F1 plants) equals the entire genetic variance and this variation is passed in its entirety to the next clonal generation. This represents a major advantage over breeding progeny that requires sexual reproduction to produce the next generation.
Furthermore, all testing is conducted using clones of the F1 plants. Genotypic frequencies do not change since there is no inbreeding. Gene frequencies change only with further crossing. The genotype finally released as a new cultivar is accessible to the breeder immediately after the initial crossings!
Why Further Steps Are Needed
Theoretically, it is possible to identify the “best” clones in the first year of testing. However, there are obstacles to accomplishing this:
- Plants grown from seed do not perform comparably with plants generated from vegetative planting material. Furthermore, the plants raised from seed are typically grown in the greenhouse, which is not representative of field conditions.
- Propagation of materials for testing takes time. The amount of planting materials available at each stage is determined by the propagation coefficient of the crop. Potato has a relatively low propagation coefficient of approximately 10 (compared to sweet potato at 30+).
- Clonally propagated crops often involve complex product targets. Many traits are required at the farmer, processor, and consumer levels. Evaluations to facilitate selection are numerous and require significant amounts of testing materials.
- Genotype by environment interaction (GxE) can be very high for yield and other low heritable traits in clonally propagated crops like potato. Multiple-location, multiple-year evaluation for yield, disease/pest resistances, and quality traits are needed to satisfy product demands locally and regionally.
Examining Genotype by Environment by Management (GxExM) Systems
In addition, because all genetic variation (additive, dominance, epistatic) is captured in clonally propagated crops, GxE can be examined in depth to identify “best” genotypes for specific types of environments.
After all, phenotype (P) is the observed expression of a trait in an individual, as determined by genetic makeup and environmental factors.
[latex]P = f(G, E, GxE)[/latex]
where
G = genetic effects
E = environmental effects (locations, years, seasons, etc.)
GxE = genotype x environment interaction effects
For example, phenotypic expression between clones of the same genotype grown in two different environments becomes a function of environmental variation. (Note: with only one genotype considered, GxE is not relevant or discernable.)
To explore the environmental effects and their impact on phenotype further, the particular management systems and practices to be employed in crop production must be detailed.
[latex]P = f(G, E, M, GxE, GxM, ExM, GxExM)[/latex]
Genotype by environment by management (GxExM) system can be exploited to realize the full genetic potential of new cultivars in crop production.
More on Genotype by Environment by Management (GxExM)
What aspects of the management system contribute to GxExM?
Some relevant management aspects include:
- Watering regime
- Tillage regime
- Soil fertility regime
- Pest control regimes
- Plant density
- Sowing date
- Row spacing
All of these aspects have the potential to influence productivity and quality of crop production.
For potato, an important element of cultivar release is guidance to farmers on management aspects of the new cultivar in production. Thus, management practices must be addressed in the testing regime prior to new cultivar release, e.g., ridge-furrow tillage system (Fig. 3).

Complex Product Targets
Target traits for potato must meet demands of the value chain stakeholders and may include:
- High fresh-weight yield
- Yield stability
- Abiotic stress tolerances to low soil fertility, drought, heat, salinity
- Maturity
- Tuber appearance: number, shape, size, uniformity, skin color, flesh color, eye depth, lack of internal defects (e.g. hollow heart, brown center), lack of external defects (e.g., cracks, greening)
- Adaptation to local environment
- Disease resistance (e.g. late blight, early blight, bacterial wilt, potato leafroll virus, etc.)
- Pest tolerances (e.g. Potato tuber moth, green peach aphid, leafminer fly, nematodes)
- Flavor, aroma, texture
- Nutrient and anti-nutrient content (e.g. carotenoids, anthocyanins, Vitamin C, micronutrients; low levels of glycoalkaloids)
- Storage life
- Processing qualities (e.g. frying for French fries or chips, blackening after cooking)
- Starch type (altered ratio of amylopectin to amylose for industrial purposes)
See Bradshaw and Bonierbale (2010) for a comprehensive list of potential traits of interest.
Product targets are typically complex and require many types of screens to evaluate performance. Because of the fresh produce market for potato, it is critical to have farmers and consumers participate early in the selection process. However, farmer participation is best limited to selection for visual traits, especially those for which a particular threshold for performance is essential. Lowly heritable traits like yield are best selected by the breeder.
Choosing Parents
What are the priorities for choosing parents to achieve a complex breeding target in potato?
The goal of breeding crosses is to create breeding populations with high mean performance for traits of interest as well as wide genetic variability. Again, considering all traits of interest, the ideal is a good x good cross, resulting in new combinations of favorable alleles and more loci with the favorable genotype.
For autotetraploid, highly heterozygous potato, per se performance of parents is not necessarily a good indicator of breeding value; combining ability may be more informative. In this situation, the GCA (general combining ability) for a quantitative trait is composed of additive and additive-by-additive epistatic gene effects. For traits involving heterosis (e.g. yield), SCA (specific combining ability) may be critical to trait performance in progeny. Thus, diallel analysis may be useful in estimating breeding value (see Chapter 3 for guidelines and ALA example) and more reliable than per se performance in choosing parents.
Commonly, the parents include a set of 100+ clones selected in the previous year following preliminary trials for all traits. Thus, a form of recurrent selection may be practiced.
Mating Designs and Breeding Methods
Due to the prominence of self-incompatibility among clonally propagated crops, the polycross procedure is commonly used to create F1 progeny from multiple parents. The goal is to obtain equal genetic contribution from each parent among the progeny created.
For the polycross design to be an effective means of intercrossing multiple parents:
- Parents must be physically positioned such that there is equal chance for all possible crosses to be made.
- Parents must be flowering at the same time.
- Procedures for bulking resulting seed must facilitate equal genetic contribution of each parent.
Two experimental designs are especially useful for arranging the parents in the field to facilitate random mating.
- The Latin Square (Fig. 4), which arranges each parent adjacent to every other parent across the replicated plantings. More than one Latin Square can be used to increase the number of replications.
- The Randomized Complete Block (RCB) design (Fig. 5), which does not facilitate proximity of all parental pairs but does offer opportunity for more replications and can handle higher numbers of parents than the Latin Square.
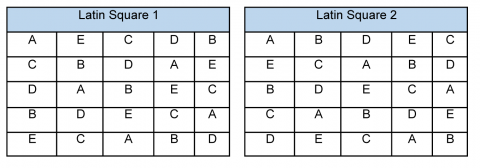
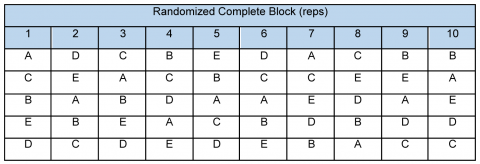
Of course, biparental crosses can also be arranged to produce progeny instead of a polycross approach.
Producing Breeding Cross Progeny

To promote flowering of difficult clones, potatoes may be grafted onto rootstocks of tomato (a related species), which may improve the amount and duration of flowering and reduce abortion of unpollinated or newly pollinated flowers (Fig. 6).
Once F1 seed is generated, it can be bulked in a number of ways:
- Across the parent plants
- By parent, with an equal amount of seed from each parent bulked
- By parent, with an equal amount of seed from each replication of each parent and an equal amount of seed from each parent bulked. This approach not only ensures an equal amount of seed of each parent but a similar genetic contribution of each parental pair. Across each replication, a parent would be in physical proximity to different other plants contributing pollen.
Example Commercial Potato Cultivar Improvement Program
Consider a suitable testing regime to identify improved cultivars of a clonally propagated crop that meets a specific complex product target, using a commercial potato breeding program as an example (Table 1).
| Season | Activity |
|---|---|
| 1 | Cross parent lines to generate 150-200 populations; true seed is created (~200 seeds per fruit) |
| 2 | Grow true seed in greenhouse; a single tuber is harvested from each of 30,000-40,000 seedlings. No selection except perhaps against disease-infected plants. |
| 3 | Grow tubers in single row plots with 3-5 plants in unreplicated trial at 1-2 locations. Visually select against undesirable traits: e.g. plant type, excessively late maturity, unacceptable tuber type. |
| 4 | Grow >5,000 selected clones in 2-3 row plots at ≥2 locations (one may be a disease screening site).
1. Select visually for highly heritable traits (e.g. color, traits with threshold requirements) 2. Select visually (or with ELISA) for disease/pest resistances with farmer participation 3. Select for yield and quality traits |
| 5 | Grow 100-200 selected clones in 3-5 row plots at multiple locations including marginal or high stress environment. Evaluate yield and all agronomic traits including yield stability as well as post-harvest traits and quality traits; farmer participation in selection for visual traits. |
| 6 | Evaluate 50-100 selected clones for yield, agronomic, quality, post-harvest traits in 5-row plots at multiple locations; farmer participation, particularly for flavor and post-harvest; best clones coded. |
| 7 | Advanced replicated yield trials by maturity group in many locations. |
| 8 | 1. Advanced replicated yield trials by maturity group in many locations. Disease and quality screening continues.
2. Agronomic trials to develop crop management specifications. 3. S-clone propagation (“seed clones” produced as a source of planting material) and in vitro maintenance of disease-free true seed plantlets. |
| 9 | On-farm trials and large-scale grower trials; evaluate consumer acceptance as fresh or processed product. |
| 10 | Variety registration |
| 11 | Release 0-2 new varieties |
Salient Features
We observe the following with respect to the Example Commercial Potato Cultivar Improvement Program:
- A large number of crosses are made, with at least 200 progeny per cross.
- The “true seed” is grown in the greenhouse to establish the F1 plants that represent the families in the breeding crosses.
- The first selection focuses on elimination of undesired genotypes (not selection for top performance) with moderately high selection intensity.
- Early testing stages focus on discarding undesirables, selecting for high-heritability traits and those that can be scored visually; advanced testing stages focus on selection for yield and other lowly heritable traits, while continuing selection for other key traits like disease resistance.
- Plot sizes increase and testing locations expand as testing advances with selected clones.
- Farmers are engaged early in participatory breeding and throughout the selection process, especially to provide input on visually-scored traits, flavor, and post-harvest characteristics.
- Selection approaches may include independent culling (if a clone does not meet selection thresholds for one trait, it is discarded in other tests preferably before harvest); index selection facilitates selection in a complex product target for multiple traits that may be negatively correlated with yield.
- The selected clones from Season 6 are “coded” and progressed to advanced testing at numerous locations.
- Yield and other lowly heritable traits influenced by GxE are evaluated for multiple years in multiple locations.
- S-clones of selections advanced through regional trials are propagated in concert with advanced testing to prepare for release and distribution of potential new varieties. A disease-free source for S-clone propagation must be maintained.
- Commercial grower trials provide evaluation of bulk handling and consumer/processor acceptance as a fresh or processed product.
- Plant materials for testing can originate with the previous year’s trials unless there is a disease build-up.
How does this breeding and testing regime differ from one focused on development of improved single-cross corn hybrids?
More About S-clones

With clonally propagated crops, the source of planting materials for cultivar production by farmers is not seed. It is the clonally propagated plant part (e.g., tubers) of the new, improved variety. For potato, these source planting materials are tubers referred to as S-clones or “seed clones” (Fig. 7). Because sexual reproduction has no place in maintaining the S-clones, there is no risk of contaminating the new, improved variety due to pollen migration. The only genetic changes to the variety would occur through mutation, which is rare. However, disease transmission is a risk as many diseases can be spread through vegetative plant parts.
A newly developed variety can be lost without adequate safeguarding from disease infection. After all, a clone developed from two heterozygous parents cannot be reproduced by crossing the parents again. Virus diseases are particularly threatening since viruses cannot be controlled chemically and are often spread by insect vectors. Thus, to have a source of healthy base material, true seed plantlets may be maintained in vitro under disease-free conditions until final selections are made from each batch. This virus-free starter material can be used to replace S-clones of new, improved varieties that are overcome in field environments. It is the breeder’s responsibility to provide disease-free starter materials for S-clone propagation as part of the hand-off to Supply Chain (the core function in the process pipeline charged with producing materials for distribution of new varieties to farmers).

Besides potato, other crops that are typically maintained and distributed for cultivation by asexual reproduction include the below (some shown in Figs. 8 and 9):
- Cassava
- Sweet potato
- Yam
- Taro
- Banana
- Plantain
- Sugar cane
- Apple
- Strawberry
- Grapes

References
Bradshaw, J.E., and M. Bonierbale. 2010. Potatoes. In J.E. Bradshaw (ed). Handbook of Plant Breeding: Root and Tuber Crops. Springer Science+Business Media, LLC, New York.
How to cite this chapter: Mumm, R.H. (2023). New Line Development and New Line Evaluation Clonally Propagated Cultivars. In W. P. Suza, & K. R. Lamkey (Eds.), Cultivar Development. Iowa State University Digital Press.
Organisms that are genetically identical to their progenitor.
(1) Plants comprising the population are genetically identical.
(2) Population is comprised of genetically identical plants.
The polycross is a method of intercrossing lines by natural hybridization used especially with species having mechanisms that prevent self-pollination.
"Seed clones" produced as a source of planting material.

