Physical Development in Infancy and Toddlerhood
Diana Lang; Alisa Beyer; Julie Lazzara; Suzanne Valentine-French; Martha Lally; and Naomi H. Dan Karami
- Summarize overall physical growth during infancy. Compare gross and fine motor skills and give examples of each.
- Describe the growth of the brain during infancy.
- Discuss nutritional concerns of marasmus and kwashiorkor.
- Describe cognitive development in infancy and toddlerhood. Describe the six substages of sensorimotor intelligence, infant memory, and language development.
- Describe stages of language development during infancy. Define babbling, holophrastic speech, and overregularization.
- Contrast styles of attachment.
- Discuss the importance of temperament and goodness of fit.
- Describe self-awareness, stranger wariness, and separation anxiety.
- Use Erikson’s theory to characterize psychosocial development during infancy.
We will now turn our attention to the physical, cognitive, and socioemotional development during the first two years. Researchers have given this part of the lifespan more attention than any other period, perhaps because changes during this time are so dramatic and so noticeable. We have also assumed that what happens during these years provides a foundation for one’s life to come. However, it has been argued that the significance of development during these years has been overstated.[1]
Physical Growth and Development
The average newborn in the United States weighs about 7.5 pounds (between 5 and 10 pounds) and is about 20 inches in length. For the first few days of life, infants typically lose about 5 percent of their body weight as they eliminate waste and get used to feeding. This often goes unnoticed by most parents but can be cause for concern for those who have a smaller infant. This weight loss is temporary, however, and is followed by a rapid period of growth. By the time an infant is 4 months old, it usually doubles in weight and by one year has tripled the birth weight. By age 2, the weight has quadrupled, so we can expect that a 2-year-old should weigh between 20 and 40 pounds. The average length at one year is about 29.5 inches and at two years it is around 34.4 inches.
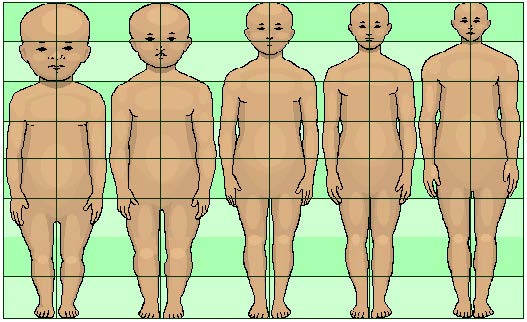
Body Proportions: Another dramatic physical change that takes place in the first several years of life is the change in body proportions. The head initially makes up about 50 percent of our entire length when we are developing in the womb. At birth, the head makes up about 25 percent of our length, and by age 25 it comprises about 20 percent our length (Figure 1).
The Brain in the First Two Years
Some of the most dramatic physical change that occurs during this period is in the brain. We are born with most of the brain cells that we will ever have; that is, about 85 billion neurons whose function is to store and transmit information.[2] While most of the brain’s neurons are present at birth, they are not fully mature. During the next several years dendrites, or branching extensions that collect information from other neurons, will undergo a period of exuberance. Because of this proliferation of dendrites, by age two a single neuron might have thousands of dendrites. Synaptogenesis, or the formation of connections between neurons, continues from the prenatal period forming thousands of new connections during infancy and toddlerhood. This period of rapid neural growth is referred to as synaptic blooming (see Figure 2).
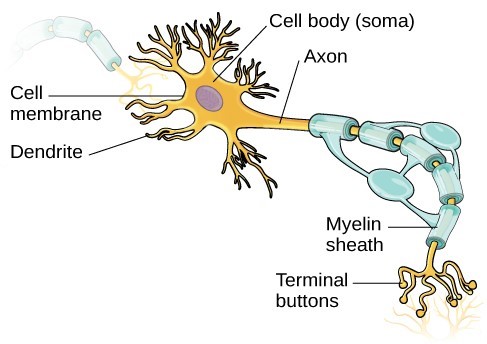
The blooming period of neural growth is followed by a period of synaptic pruning, where neural connections are reduced thereby making those that are used much stronger. It is thought that pruning causes the brain to function more efficiently, allowing for mastery of more complex skills.[3] Experience will shape which of these connections are maintained and which of these are lost. Ultimately, about 40 percent of these connections will be lost.[4] Blooming occurs during the first few years of life, and pruning continues through childhood and into adolescence in various areas of the brain.
Another major change occurring in the central nervous system is the development of myelin, a coating of fatty tissues around the axon of the neuron.[5] Myelin helps insulate the nerve cell and speed the rate of transmission of impulses from one cell to another. This enhances the building of neural pathways and improves coordination, control of movement, and thought processes. The development of myelin continues into adolescence, but is most dramatic during the first several years of life.
At birth, the brain is about 25 percent its adult weight and by age two years it is at 75 percent its adult weight. Most of the neural activity is occurring in the cortex or the thin outer covering of the brain involved in voluntary activity and thinking. The cortex has two hemispheres and each hemisphere is divided into four lobes, each separated by folds known as fissures. If we look at the cortex starting at the front of the brain and moving over the top (see Figure 3), we see first the frontal lobe (behind the forehead), which is responsible primarily for thinking, planning, memory, and judgment. Following the frontal lobe is the parietal lobe, which extends from the middle to the back of the skull and which is responsible primarily for processing information about touch. Next is the occipital lobe, at the very back of the skull, which processes visual information. Finally, in front of the occipital lobe, between the ears, is the temporal lobe, which is responsible for hearing and language.
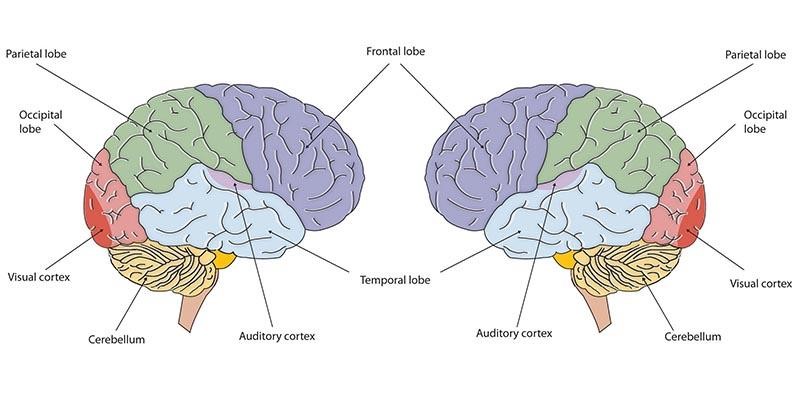
Although the brain grows rapidly during infancy, specific brain regions do not mature at the same rate. Primary motor areas develop earlier than primary sensory areas, and the prefrontal cortex, that is located behind the forehead, is the least developed. As the prefrontal cortex matures, the child is increasingly able to regulate or control emotions, to plan activities, strategize, and have better judgment. This is not fully accomplished in infancy and toddlerhood, but continues throughout childhood, adolescence, and into adulthood.
Lateralization is the process in which different functions become localized primarily on one side of the brain. For example, in most adults the left hemisphere is more active than the right during language production, while the reverse pattern is observed during tasks involving visuospatial abilities[6]. This process develops over time, however, structural asymmetries between the hemispheres have been reported even in fetuses[7][8] and infants[9].
Lastly, neuroplasticity refers to the brain’s ability to change, both physically and chemically, to enhance its adaptability to environmental change and compensate for injury. Both environmental experiences, such as stimulation, and events within a person’s body, such as hormones and genes, affect the brain’s plasticity. So too does age. Adult brains demonstrate neuroplasticity, but they are influenced more slowly and less extensively than those of youth.[10]
Infant Sleep
A newborn typically sleeps approximately 16.5 hours per 24-hour period. This is usually polyphasic sleep in that the infant is accumulating the 16.5 hours over several sleep periods throughout the day[11]. The infant is averaging 15 hours per 24-hour period by one month, and 14 hours by 6 months. By the time children turn two, they are averaging closer to 10 hours per 24 hours.
Additionally, the average newborn will spend close to 50% of the sleep time in the Rapid Eye Movement (REM) phase, which decreases to 25% to 30% in childhood.
Sudden Unexpected Infant Deaths (SUID): Each year in the United States, there are about 3,400 Sudden Unexpected Infant Deaths (SUID).[12] These deaths occur among infants less than one year-old and have no immediately obvious cause. The three commonly reported types of SUID are:
Sudden Infant Death Syndrome (SIDS): SIDS is identified when the death of a healthy infant occurs suddenly and unexpectedly, and medical and forensic investigation findings (including an autopsy) are inconclusive. SIDS is the leading cause of death in infants 1 to 12 months old within the United States, and approximately 1,250 infants died of SIDS in 2019.[13]
Because SIDS is diagnosed when no other cause of death can be determined, possible causes of SIDS are regularly researched. One leading hypothesis suggests that infants who die from SIDS have abnormalities in the area of the brainstem responsible for regulating breathing (Weekes-Shackelford & Shackelford, 2005).
Unknown Cause: The sudden death of an infant less than one year of age that cannot be explained because a thorough investigation was not conducted and cause of death could not be determined.
Accidental Suffocation and Strangulation in Bed: Reasons for accidental suffocation include: Suffocation by soft bedding, another person rolling on top of or against the infant while sleeping, an infant being wedged between two objects such as a mattress and wall, and strangulation such as when an infant’s head and neck become caught between crib railings.
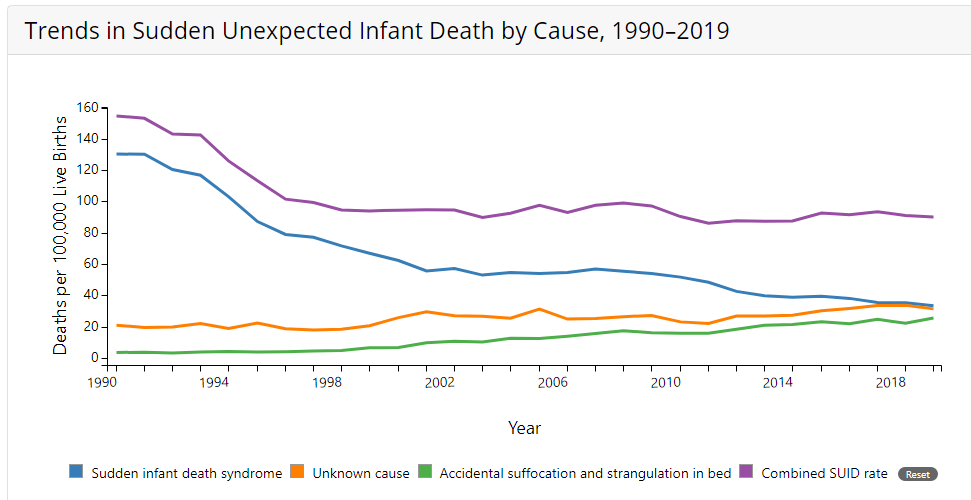
As can be seen in Figure 4, the combined SUID death rate declined considerably in the United States following the release of the American Academy of Pediatrics safe sleep recommendations in 1992, which advocated that infants be placed for sleep on their backs (nonprone position). These recommendations were followed by a major Back to Sleep Campaign in 1994.
Should infants share the bed with another person? Researchers analyzed a total of 8207 deaths from 24 states during 2004–2012 that were contained in the National Center for the Review and Prevention of Child Deaths Case Reporting System, a database of death reports from state child death review teams.[14] The results indicated that younger victims (0-3 months) were more likely to die by bed-sharing and sleeping in an adult bed/on a person. A higher percentage of older victims (4 months to 364 days) rolled into objects in the sleep environment and changed position from side/back to prone. Investigators compared infants who died of SIDS with a matched control and found that infants younger than three months old who slept in bed with a parent were five times more likely to die of SIDS compared to babies who slept separately from the parents, but were still in the same room.[15]
They concluded that bed sharing, even when the parents do not smoke or take alcohol or drugs, increases the risk of SIDS. However, when combined with parental smoking and maternal alcohol consumption and/or drug use, risks associated with bed sharing greatly increased.
The two studies discussed above were based on American statistics. What about the rest of the world? Co-sleeping occurs in many cultures, primarily because of a more collectivist perspective that encourages a close parent-child bond and interdependent relationship (Morelli, Rogoff, Oppenheim, & Goldsmith, 1992). In countries where co-sleeping is common, however, parents and infants typically sleep on floor mats and other hard surfaces which minimize the suffocation that can occur with bedding and mattresses.[16]
From Reflexes to Voluntary Movements
Newborns are equipped with a number of reflexes, which are involuntary movements in response to stimulation. These movements occur automatically and are signals that the infant is functioning well neurologically. Some of the more common reflexes, such as the sucking reflex (infants suck on objects that touch their lips automatically) and rooting reflex, are essential to feeding. The grasping and stepping reflexes are eventually replaced by more voluntary behaviors. Within the first few months of life, these reflexes disappear, while other reflexes, such as the eye-blink, swallowing, sneezing, gagging, and withdrawal reflex stays with us as they continue to serve essential functions. Reflexes offer insight into the maturation and health of the nervous system. Reflexes that persist too long may impede healthy development.[17][18] In preterm infants and those with neurological impairments, some of these reflexes may be absent at birth. Once present, they may persist longer than in a neurologically healthy infant.[19]
Motor Development
Motor development occurs in an orderly sequence as infants move from reflexive reactions (e.g., sucking and rooting) to more advanced motor functioning. As mentioned during the prenatal section, development occurs according to the cephalocaudal (from head to tail) and proximodistal (from the midline outward) principles. For instance, babies first learn to hold their heads up, then to sit with assistance, then to sit unassisted, followed later by crawling, pulling up, cruising, and then walking. As motor skills develop, there are certain developmental milestones that young children should achieve. For each milestone there is an average age, as well as a range of ages in which the milestone should be reached. An example of a developmental milestone is a baby holding up its head. Babies on average are able to hold up their head at 6 weeks old, and 90% of babies achieve this between 3 weeks and 4 months old. When babies are not holding up their head by 4 months old, they are typically showing a delay. On average, most babies sit alone at 7 months old. Sitting involves both coordination and muscle strength, and 90% of babies achieve this milestone between 5 and 9 months old. If the child is displaying delays on several milestones, that is reason for concern, and the parent or caregiver should discuss this with the child’s pediatrician. Some developmental delays can be identified and addressed through early intervention.
Motor Skills refer to our ability to move our bodies and manipulate objects. Fine motor skills focus on the muscles in our fingers, toes, and eyes, and enable coordination of small actions (e.g., grasping a toy, writing with a pencil, and using a spoon). Newborns cannot grasp objects voluntarily but do wave their arms toward objects of interest. At about 4 months of age, the infant is able to reach for an object, first with both arms and within a few weeks, with only one arm. At this age grasping an object involves the use of the fingers and palm, but no thumbs. This is known as the palmar grasp. The use of the thumb comes at about 9 months of age when the infant is able to grasp an object using the forefinger and thumb. Now the infant uses a pincer grasp, and this ability greatly enhances the ability to control and manipulate an object and infants take great delight in this newfound ability. They may spend hours picking up small objects from the floor and placing them in containers. By 9 months, an infant can also watch a moving object, reach for it as it approaches, and grab it.
Gross motor skills focus on large muscle groups that control our head, torso, arms and legs and involve larger movements (e.g., balancing, running, and jumping). These skills begin to develop first. Examples include moving to bring the chin up when lying on the stomach, moving the chest up, and rocking back and forth on hands and knees. However, it also includes exploring an object with one’s feet as many babies do as early as 8 weeks of age if seated in a carrier or other device that frees the hips. This may be easier than reaching for an object with the hands, which requires much more practice.[20] Sometimes an infant will try to move toward an object while crawling and surprisingly move backward because of the greater amount of strength in the arms than in the legs.
Sensory Capacities
Throughout much of history, the newborn was considered a passive, disorganized being who possessed minimal abilities. However, current research techniques have demonstrated just how developed the newborn is with especially organized sensory and perceptual abilities.
Vision
The womb is a dark environment void of visual stimulation. Consequently, vision is the most poorly developed sense at birth and time is needed to build those neural pathways between the eye and the brain. Newborns typically cannot see further than 8 to 16 inches away from their faces, and their visual acuity is about 20/400, which means that an infant can see something at 20 feet that an adult with normal vision could see at 400 feet. Thus, the world probably looks blurry to young infants. Because of their poor visual acuity, they look longer at checkerboards with fewer large squares than with many small squares. Infants’ thresholds for seeing a visual pattern are higher than adults. Thus, toys for infants are sometimes manufactured with black and white patterns rather than pastel colors because the higher contrast between black and white makes the pattern more visible to the immature visual system. By about 6 months, infants’ visual acuity improves and approximates adult 20/25 acuity.
When viewing a person’s face, newborns do not look at the eyes the way adults do; rather, they tend to look at the chin a less detailed part of the face. However, by 2 or 3 months, they will seek more detail when exploring an object visually and begin showing preferences for unusual images over familiar ones, for patterns over solids, for faces over patterns, and for three-dimensional objects over flat images. Newborns have difficulty distinguishing between colors, but within a few months they are able to discriminate between colors as well as adults. Sensitivity to binocular depth cues, which require inputs from both eyes, is evident by about 3 months and continues to develop during the first 6 months. By 6 months, the infant can perceive depth perception in pictures as well.[21] Infants who have experience crawling and exploring will pay greater attention to visual cues of depth and modify their actions accordingly.[22]
Hearing
The infant’s sense of hearing is very keen at birth, and the ability to hear is evidenced as soon as the 7th month of prenatal development. In fact, an infant can distinguish between very similar sounds as early as one month after birth and can distinguish between a familiar and non-familiar voice even earlier. Infants are especially sensitive to the frequencies of sounds in human speech and prefer the exaggeration of infant-directed speech, which will be discussed later. Additionally, infants are innately ready to respond to the sounds of any language, but some of this ability will be lost by 7 or 8 months as the infant becomes familiar with the sounds of a particular language and less sensitive to sounds that are part of an unfamiliar language.
Touch and Pain
Immediately after birth, a newborn is sensitive to touch and temperature, and is also highly sensitive to pain, responding with crying and cardiovascular responses.[23] Newborns who are circumcised, which is the surgical removal of the foreskin of the penis, without anesthesia experience pain as demonstrated by increased blood pressure, increased heart rate, decreased oxygen in the blood, and a surge of stress hormones.[24]
Taste and Smell
Studies of taste and smell demonstrate that babies respond with different facial expressions, suggesting that certain preferences are innate. Newborns can distinguish between sour, bitter, sweet, and salty flavors and show a preference for sweet flavors.
Infants seem to be born with the ability to perceive the world in an intermodal way; that is, through stimulation from more than one sensory modality. For example, infants who sucked on a pacifier with either a smooth or textured surface preferred to look at a corresponding (smooth or textured) visual model of the pacifier. By 4 months, many infants can match lip movements with speech sounds and can match other audiovisual events. Although sensory development emphasizes the afferent processes used to take in information from the environment, these sensory processes can be affected by the infant’s developing motor abilities. Reaching, crawling, and other actions allow the infant to see, touch, and organize his or her experiences in new ways.
How Infants are Tested
Habituation procedures, that is measuring decreased responsiveness to a stimulus after repeated presentations, have increasingly been used to evaluate infants to study the development of perceptual and memory skills. Phelps (2005) describes a habituation procedure used when measuring the rate of the sucking reflex.[25] Researchers first measure the initial baseline rate of sucking to a pacifier equipped with transducers that measure muscle contractions. Next, an auditory stimulus is presented, such as a human voice uttering a speech sound such as “da.” The rate of sucking will typically increase with the new sound, but then decrease to baseline levels as “da” is repeatedly presented, showing habituation. If the sound “ma” was then presented, the rate of sucking would again increase, demonstrating that the infant can discriminate between these two stimuli.
Additionally, the speed or efficiency with which infants show habituation has been shown to predict outcomes in behaviors such as language acquisition and verbal and nonverbal intelligence. Infants who show difficulty during habituation, or habituate at slower than normal rates, have been found to be at an increased risk for significant developmental delays. Infants with Down syndrome, teratogen-exposed infants, malnourished infants, and premature infants have all been studied. Researchers have found that at the age of 16 months, high-risk infants show rates of habituation comparable to newborn infants[26].
- This chapter was adapted from select chapters in Lumen Learning's Lifespan Development, authored by Martha Lally and Suzanne Valentine-French available under a Creative Commons Attribution-NonCommercial-ShareAlike license, and Waymaker Lifespan Development, authored by Ronnie Mather for Lumen Learning and available under a Creative Commons Attribution license. Some selections from Lumen Learning were adapted from previously shared content from Laura Overstreet's Lifespan Psychology. ↵
- Huttenlocher, P. R., & Dabholkar, A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology, 387(2), 167-178. ↵
- Kolb, B. & Whishaw, I. Q. (2011). An introduction to brain and behavior (3rd ed.). New York: Worth Publishers. ↵
- Webb, S. J., Monk, C. S., & Nelson, C. A. (2001). Mechanisms of postnatal neurobiological development: Implications for human development. Developmental Neuropsychology, 19, 147-171. ↵
- Carlson, N. (2014). Foundations of behavioral neuroscience (9th ed.). Boston, MA: Pearson. ↵
- Springer, S. P. & Deutsch, G. (1993). Left brain, right brain (4th ed.). New York: W. H. Freeman. Stork, F. & ↵
- Chi, J. G., Dooling, E. C., & Gilles, F. H. (1977). Left-right asymmetries of the temporal speech areas of the human fetus. Archives of Neurology, 34, 346–8. ↵
- Kasprian, G., Langs, G., Brugger, P. C., Bittner, M., Weber, M., Arantes, M., & Prayer, D. (2011). The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cerebral Cortex, 21, 1076–1083. ↵
- Dubois, J., Hertz-Pannier, L., Cachia, A., Mangin, J. F., Le Bihan, D., & Dehaene-Lambertz, G. (2009). Structural asymmetries in the infant language and sensori-motor networks. Cerebral Cortex, 19, 414–423. ↵
- Kolb, B., & Gibb, R. (2011). Brain plasticity and behaviour in the developing brain. Journal de l’Academie Canadienne de Psychiatrie de l’enfant et de l’adolescent [Journal of the Canadian Academy of Child and Adolescent Psychiatry], 20(4), 265–276. ↵
- Salkind, N. J. (2005). Encyclopedia of human development. New York: Sage Publications. ↵
- Centers for Disease Control and Prevention. (2021). About SUID and SIDS. https://www.cdc.gov/sids/about/index.htm ↵
- Centers for Disease Control and Prevention. (2021). Sudden Unexpected Infant Death and Sudden Infant Death Syndrome - Data and Statistics. https://www.cdc.gov/sids/data.htm ↵
- Colvin, J.D., Collie-Akers, V., Schunn, C., & Moon, R.Y. (2014). Sleep environment risks for younger and older infants. Pediatrics Online. http://pediatrics.aappublications.org/content/pediatrics/early/2014/07/09/peds. 2014-0401.full.pdf ↵
- Carpenter, R., McGarvey, C., Mitchell, E. A., Tappin, D. M., Vennemann, M. M., Smuk, M., & Carpenter, J. R. (2013). Bed sharing when parents do not smoke: is there a risk of SIDS? An individual level analysis of five major case-control studies. BMJ Open, 3(5), e002299. https://doi.org/10.1136/bmjopen-2012-002299 ↵
- Nelson, E. A., Schiefenhoevel, W., & Haimerl, F. (2000). Child care practices in nonindustrialized societies. Pediatrics, 105(6), E75. https://doi.org/10.1542/peds.105.6.e75 ↵
- Berne, S. A. (2006). The primitive reflexes: Considerations in the infant. Optometry & Vision Development, 37(3), 139-145. ↵
- Bloem, M. (2007). The 2006 WHO child growth standards. BMJ : British Medical Journal, 334(7596), 705–706. ↵
- El-Dib, M., Massaro, A. N., Glass, P., & Aly, H. (2012). Neurobehavioral assessment as a predictor of neurodevelopmental outcome in preterm infants. Journal of Perinatology, 32, 299-303. ↵
- Berk, L. (2007). Development through the life span (4th ed.). Boston: Allyn and Bacon. ↵
- Sen, M. G., Yonas, A., & Knill, D. C. (2001). Development of infants’ sensitivity to surface contour information for spatial layout. Perception, 30, 167-176. ↵
- Berk, L. E. (2007). Development through the life span (4th ed.). Boston: Allyn and Bacon. ↵
- Balaban, M. T. & Reisenauer, C. D. (2013). Sensory development. In N. J. Salkind (Ed.), Encyclopedia of human development (pp. 1144-1147). New, York: Sage Publications. ↵
- United States National Library of Medicine. (2016). Circumcision. https://medlineplus.gov/circumcision.html ↵
- Phelps, B. J. (2005). Habituation. In N. J. Salkind (Ed.), Encyclopedia of human development (pp. 597-600). New York: Sage Publications. ↵
- Phelps, B. J. (2005). Habituation. In N. J. Salkind (Ed.), Encyclopedia of human development (pp. 597-600). New York: Sage Publications. ↵

