Molecules of Life: The Central Dogma and RNA Vaccines
Development of the SARS-CoV-2 mRNA vaccine
Karri Haen Whitmer
Normally, the pre-market processes required to produce a vaccine for a novel disease take years to complete (Figure 1). These processes of vaccine development include exploratory research, pre-clinical testing, clinical trials, review and approval, manufacturing and quality control. In the U.S., there are three phases of human clinical trials: Phase 1.) the vaccine is given to small groups of people to test for safety and dosage; Phase 2.) a broader array of the population receives the vaccine (hundreds of people) to further refine dosage, safety, and to test for immunogenicity; and Phase 3.) thousands of people receive the vaccine and the vaccine is rigorously tested for efficacy against infection. When all three phases have been completed, the U.S. Food and Drug Administration (FDA) reviews the safety and efficacy of the vaccine before it can go to market.
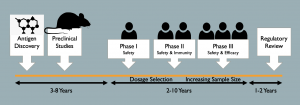
Even the most expediently developed vaccines have taken 12-18 months to be created, tested, and approved. The Pfizer-BioNTech and Moderna vaccines developed for the SARS-CoV-2 virus approved for the treatment of Covid-19 took much less time to get to market (approximately 10 months from vaccine candidate identification to regulatory review and dispersal). What are some of the reasons behind this unusually short vaccine development timeline?
Besides the emergency caused by the pandemic crisis, there are several factors that allowed researchers to develop a novel vaccine against Covid-19 much faster than typical. For example, there was abundant prior knowledge about the role of the viral spike protein, a glycoprotein antigen that allows the virus to enter cells, in other coronavirus diseases. There was also evidence that neutralizing antibodies, antibodies that bind to a virus, preventing it from interacting with a host cell, are important to achieving coronavirus immunity.
Even with good prior knowledge of what viral antigens will provoke an immune response, traditional vaccine development takes six months to produce, treat, and purify virus grown inside fertilized chicken eggs. This is the technique used for the seasonal influenza vaccine. The Pfizer-BioNTech and Moderna vaccines. The greatest reason the first COVID-19 vaccines were developed so quickly is the type of molecule utilized in creating the vaccine: the Moderna and Pfizer SARS-CoV-2 vaccines are mRNA vaccines, vaccines that deliver a small molecule called messenger RNA, which encodes a gene for virus surface protein, to the host’s body cells. The Moderna vaccine was already being administered in Phase 1 clinical trials a mere 10 weeks after the genetic sequence of the virus was released, and the Pfizer vaccine is now the first of its kind to be approved by the FDA.
What is so special about mRNA vaccines?
Instead of delivering pieces of virus, or proteins made by the virus grown from chicken eggs, RNA vaccines deliver a synthetically manufactured genetic blueprint that allows human cells to manufacture its own, harmless viral protein. Once the protein is made in the cell’s cytoplasm, it initiates an immune response because the protein is detected as a foreign invader. This process sets the stage for a secondary exposure to SARS-CoV-2 protein in the real world.
The process of manufacturing an mRNA vaccine in the lab is a straightforward process that does not rely upon using live cells to replicate virus. Instead a simple chemical reaction can be used to create the synthetic mRNA for the virus protein target, which is then packaged in a lipid nano-droplet in order for it to be efficiently delivered to into cells. From there, your own cells do all the work. Thus, mRNA vaccines have potential to be extremely rapid, inexpensive and highly scalable solutions to vaccine development.
See more about the differences between conventional and RNA vaccines in this figure by Pfizer:
Week 3 Laboratory Activity: the Steps of COVID-19 mRNA vaccine production
In this activity, you will follow along the major steps of mRNA vaccine production and answer questions about the molecular biology of human cells. Lecture Module 1B and 1C videos found in the BIOL 256 lecture Canvas and/or Chapters 2 and 3 of the lecture text, Human Physiology, Fox, fifteenth edition, will be helpful for completing this assignment. If you are not enrolled in lecture, you may refer to OpenStax Concepts of Biology Ch. 9 (Molecular Biology) for this assignment: https://openstax.org/books/concepts-biology/pages/9-introduction
Background: You are working in a laboratory tasked with the development of the COVID-19 vaccine. Your starting data is the SARS-CoV-2 virus DNA sequence. Your laboratory supervisor informs you that you will be using the viral surface spike protein to manufacture an mRNA for the vaccine.
Your supervisor has sent you the gene sequence for the mRNA that is to be synthesized for the vaccine. You wish to check the accuracy of the sequence against a database before you get to work. You will need to use bioinformatics to find this out.
The DNA sequence is below:
atgtttgtttttcttgttttattgccactagtctctagtcagtgtgttaatcttacaaccagaactcaattaccccctgcatacactaattctttcacacgtggtgtttattaccctgacaaagttttcagatcctcagttttacattcaactcaggacttgttcttacctttcttttccaatgttacttggtaccatgctatacatgtctctgggaccaatggtactaagaggtttgataaccctgtcctaccatttaatgatggtgtttattttgcttccactgagaagtctaacataataagaggctggatttttggtactactttagattcgaagacccagtccctacttattgataataacgctactaatgttgatattaaagtctgtgaattacaactttgtaatgatacatttttgggtgtttattaccacaaaaacaacaaaagttggatggaaagtgagttcagagtttattctagtgcgaataatagcacttttgaatatgtctctcagccttttcttatggaccttgaaggaaaacagggtaatttcaaaaatcttagggaatttgtgtttaagaatattgatggttattttaaaatatattctaagcacacgcctattaatttagtgcgtgatctccctcagggtttttcggctttagaaccattggtagatttgccaataggtattaacatcactaggtttcaaactttacttgctttacatagaagttatttgactcctggtgattcttcttcaggttggacagctggtgctgcagcttattatgtgggttatcttcaacctaggacttttctattaaaatataatgaaaatggaaccattacagatgctgtagactgtgcacttgaccctctctcagaaacaaag
Copy the DNA sequence.
Next, go to https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch
Paste the sequence into the yellow text box.
Scroll to the bottom of the page and click BLAST.
Note that the search may take a while as it compares the DNA sequence you entered to all the other DNA sequences in the database.
Question 1. When the search result loads, scroll down to “Sequences producing significant alignments” under the descriptions tab. What is the name of the best match for this sequence? You only need to copy the name from the top entry in the list. You may need to click the link to see the complete title.
Question 2. The sense-strand DNA sequence of the protein gene begins as follows. Replicate the anti-sense strand for this DNA on the line.
5’ atg ttt gtt ttt ctt gtt tta ttg cca cta gtc tct agt 3’
3’ ___________________________________5’
Question 3. Transcribe the antisense DNA strand you created (above) into a mRNA molecule. Do not forget to write the 5’-3’ directionality on the molecule.
mRNA:
Question 4. What are the 5’ (cap) and 3’ (tail) modifications that are used to make mRNA more stable inside animal cells?
5’ modification:
3’ modification:
Question 5. Add a poly-A tail to your mRNA sequence from question 3 (10 or more adenine nucleotides).
mRNA:
Question 6. Where in the animal cell are proteins translated?
Question 7. What is the delivery vehicle of the Moderna vaccine that protects the vaccine mRNA as well as delivers it into human cells?
Question 8. What properties of the plasma membrane allow the vaccine vehicle to dispense the synthetic mRNA into a human cell?
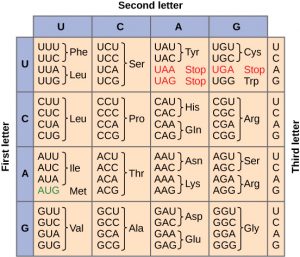
Question 9. Using the standard genetic code table (Figure 2), translate the mRNA message from Question 4, above. Note, the poly-A tail is not translated into protein.
mRNA sequence (question 3):
Protein sequence:
Please cite:
Haen Whitmer, K.M. (2021). A Mixed Course-Based Research Approach to Human Physiology. Ames, IA: Iowa State University Digital Press. https://iastate.pressbooks.pub/curehumanphysiology/

