Phyllosilicate Colloids and their Surface Chemistry
Rivka Fidel
Upon completing this chapter, you should be able to:
- Define phyllosilicates
- Explain:
- the difference between colloids, clays and phyllosilicates
- the difference between a 2:1 and 1:1 phyllosilicate
- what happens when phyllosilicates swell and why swelling is important
- where phyllosilicates get their surface charges from
- Match types of charges (permanent and pH-dependent) with their definitions and where they come from
Review of Colloid Types
Recall from the previous chapter that colloids are all particles less than 1 micrometer in diameter, and that there are 4 types:
- Crystalline silicate clays (phyllosilicates): layered minerals containing SiO4
- Poorly crystalline silicates: sphere and tube-shaped minerals containing SiO4
- Oxides and hydroxides: highly weathered minerals containing O2-, OH–, and OOH3-, bonded to transition and post-transition metals (mainly Fe and Al)
- Organic colloids: particles of biological origin <1 μm in size that contain C-H and/or C-C bonds, also called “colloidal humus”
Here we will focus on the first type, crystalline silicate clays. The remaining types will be discussed in the next chapter.
Crystalline Silicate Clays (Phyllosilicates)
Crystalline silicate clays are the most abundant colloids in most soils, and have a disproportionate effect on soil properties. We’ve learned that the term “clay” can refer to any mineral particle less than 2 µm in diameter. The majority of these clay particles are crystalline silicate clays, also known as phyllosilicates. From Greek, “phyllo-“ refers to layer, and “silicate” refers to SiO42-. As reflected in their two names, these important minerals have 3 main properties:
- Crystalline: all phyllosilicates have atoms arranged in a regular, near-infinitely repeating structure
- Layers: the crystal structure of phyllosilicates is arranged in flat, paper-like layers
- Silicate: phyllosilicates all contain silicate tetrahedra. Each tetrahedron has the formula SiO42-, but since oxygens are shared, the stoichiometric formula of silicate is frequently written as SiO2 (this is the formula of quartz for example, the dominant mineral in most sand particles).

Phyllosilicate sheets and layering patterns
Phyllosilicates, also known as “layer clays,” can be classified according to their layering pattern and chemical composition. Layering patterns are defined by how tetrahedral and octahedral sheets are arranged.
Tetrahedral sheets are composed primarily of silicate tetrahedra, SiO42-. In each tetrahedron, the Si is at the center, and is bonded to 4 oxygens arranged in a pyramid-like shape with a triangular base. These can be drawn in various ways.
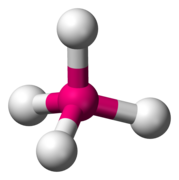
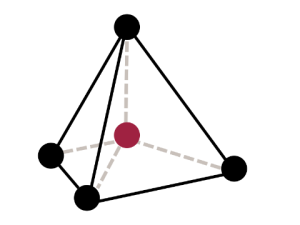
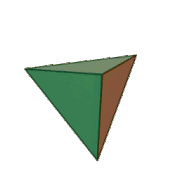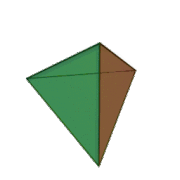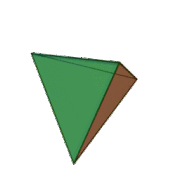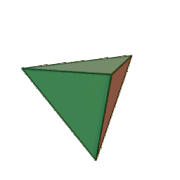
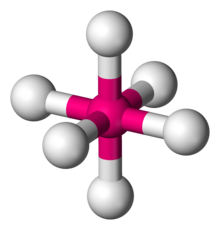
Octahedral sheets are composed primarily of aluminum and magnesium octahedra. In each octahedron, the aluminum or magnesium is located at the center, and is bonded to 8 oxygens. The overall shape resembles two pyramids with square bases, fused at the base.
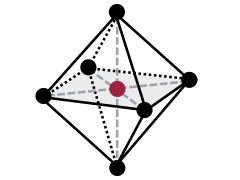
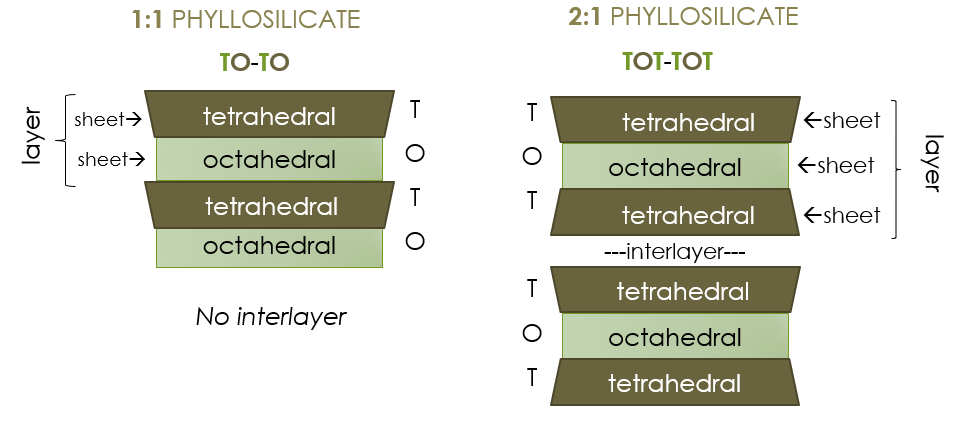
The two main patterns that these sheets arrange into as minerals form are 1:1 and 2:1. In 1:1 phyllosilicates, there is a one-to-one ratio of tetrahedral sheets to octahedral sheets. These alternate one after the other: tetrahedral, octahedral, tetrahedral, octahedral and so forth (try abbreviating this as TO-TO in your notes). Each layer contains one tetrahedral sheet and one octahedral sheet. In 2:1 phyllosilicates, there is a two-to-one ratio of tetrahedral sheets to octahedral sheets. These alternate in the order tetrahedral, octahedral, tetrahedral (try abbreviating this as TOT-TOT in your notes). Thus, each layer of a 2:1 phyllosilicate has 3 sheets. In between each 3-sheet layer is an interlayer, a space where ions and frequently also water can be found, depending on the mineral in question.
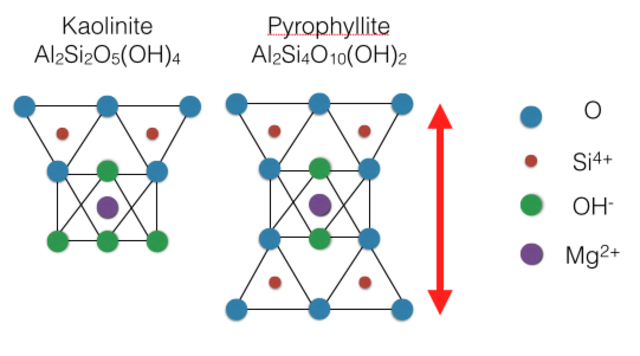
The below figure shows how atoms are arranged in these sheets of two example phyllosilicates. Notice how the oxygens in each tetrahedral sheet are shared with oxygens in the adjacent octahedral sheet.
Sources of charge in phyllosilicates
Charges of phyllosilicates and other colloids are very important because opposite charges attract. A negative surface charge will attract positively charge ions (cations), and a positive surface charge will attract negatively charged ions (anions). Many important plant nutrients like NH4+ and K+ are present as cations in soil, whereas others such as NO3– and SO42- are anions. Surface charge sites, places on colloid or clay surfaces where such ion-attracting unbalanced surface charges occur, develop on both inorganic and organic particles. Typically, these charges are only significant among clay and colloid-sized particles, whereas sand and silt contribute negligible charge due to their low surface area and chemical composition (namely their tendency to be enriched in tectosilicates like quartz and feldspar that have fewer unsatisfied charges per unit surface area).
In phyllosilicates, surface charge sites develop inside clay mineral structures during isomorphous substitution, and at crystal edges that form during physical weathering. These processes result in two different types of charge: permanent charge, and pH-dependent charge.
Permanent charge inside minerals from isomorphous substitution
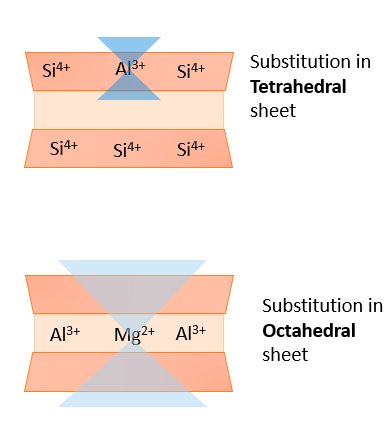
During the initial formation of phyllosilicates, a process called isomorphous substitution creates what is known as permanent charge inside phyllosilicate crystal structures. In isomorphous substitution (or isomorphic substitution), a cation of similar size but less positive charge comes in from the solution and replaces the original cation found in the mineral’s crystal structure. The name comes from “iso-” meaning same, and “-morph” meaning shape. Because this substitution occurs when a mineral first forms (not during weathering), the degree and location of isomorphous substitution is one of the defining features used to classify phyllosilicates.
In tetrahedral sheets, Al3+ commonly substitutes for Si4+. As a result, a Si tetrahedron would become an Al tetrahedron. Because Al3+ has a less positive charge than Si4+, the overall charge of the tetrahedron becomes more negative by one unit of charge. Thus, the originally neutral tetrahedron will develop an overall -1 charge.
In octahedral sheets, Mg2+ commonly substitutes for Al3+. Consequently, an Al octahedron would become a Mg octahedron. Like in the tetrahedron earlier, the Mg2+ has a less positive charge than Al3+, so the octahedron’s overall charge would decrease by 1, resulting in an overall -1 charge.
In both cases, isomorphous substitution occurs inside a sheet, and so the substituted atoms are not in contact with the solution. Thus, the charges that isomorphous substitution creates stay the same despite fluctuations in solution pH and are hence called permanent charges.
pH-dependent charge at crystal edges
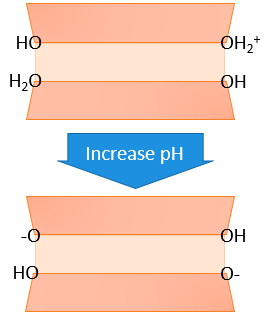
On edges, oxygens (O’s) are not shared, so without an additional bond to share electrons, charges may develop there. Essentially, cleavage of the phyllosilicate crystals at these edges breaks chemical bonds and leaves oxygens unable to share electrons with adjacent metals or metalloids (Si, Al, Mg, or Fe primarily). This creates an unsatisfied negative charge not being balanced by nearby elements. These edge charges are in contact with the solution, and hence can attract H+ ions when the pH is low. These H+ bind strongly to the surface, becoming part of the surface, and making the overall surface charge more positive (less negative). For this reason, these sites at the broken mineral edges carry what is called pH-dependent charge or variable charge. This type of charge is found in all minerals, but tends to be greatest in 1:1 phyllosilicates and oxides/hydroxides of iron and aluminum. We’ll revisit this in the chapters on cation exchange and anion exchange.
- Colloids are particles less than 1 micrometer in diameter, whereas clays are particles less than 2 micrometers in diameter
- Phyllosilicates are “layer clays”, or crystalline silicate clays. These important minerals have 3 key properties:
- Crystalline: all phyllosilicates have atoms arranged in a regular, near-infinitely repeating structure
- Layers: the crystal structure of phyllosilicates is arranged in flat, paper-like layers
- Silicate: phyllosilicates all contain silicate tetrahedra. Each tetrahedron has the formula SiO42-, but since oxygens are shared, the stoichiometric formula of silicate is frequently written as SiO2 (this is the formula of quartz for example, the dominant mineral in most sand particles).
- 2:1 phyllosilicates have (1) a tetrahedral-octahedral-tetrahedral layer pattern, and (2) an interlayer containing cations, and frequently water
- 1:1 phyllosilicates have (1) a tetrahedral-octahedral layer pattern, and (2) no interlayer
- When water enters the interlayer of select 2:1 phyllosilicates (vermiculite and smectite), they swell
- Phyllosilicates get their charges from isomorphic substitution:
- occurs when similar sized cations of different charges replace cations found in the crystal matrix
- creates permanent charge, which stays constant when pH changes and is always negative in soil
- or broken edges:
-
- charges are unsatisfied due to broken bonds
- this creates pH-dependent charge
-
- https://openpress.usask.ca/physicalgeology/chapter/5-4-silicate-minerals-2/ ↵

