Mineralogy
C. Lee Burras
- Discuss types of minerals present in the soil.
- Understand the importance of mineralogy for soil science.
- Relate mineralogy with Cation Exchange Capacity
Introduction
Soils are minerals. Even the O horizon of a Histosol is more than 70% minerals on a dry mass basis. In any other soil, minerals account for 95 to even 99.99% of the dry mass. The importance of a given mineral in soil is dependent on its prevalence and, especially, its degree of reactivity. It is important to note that soil minerals can be inherited, lost (e.g., dissolved), gained and/or moved in the profile and the landscape depending on natural and human-controlled processes.
What are minerals?
Minerals are crystalline solids. Most are naturally occurring and inorganically formed. Individual minerals (“species”) are identified and distinguished from one another based on their chemical composition and atomic framework. Restating that, a mineral cannot be uniquely identified from just its chemical composition even though on a day-to-day basis that is often how they are identified. In reality the atomic lattice is a key feature and must be known. For example, “diamond” and “graphite” are independent isomorphic mineral forms of pure “C”, yet – as everyone realizes – they have tremendously different physical and chemical properties. Their isomorphic differences are what control their hugely different value in human societies. Those difference are the function of their bonding strengths across their respective crystalline lattices – aka “atomic frameworks.”
Types of minerals
“Calcite” and “aragonite” are more mundane mineral polymorph in soils. Both are composed entirely of “CaCO3” but only calcite is the one commonly found in soil parent materials and calcic horizons. Calcite is also the one mined for ag liming and such. Aragonite is interesting in its own right, though, given it is biologically created mineral. Snails, mollusks, and other gastropods manufacture it into their shells.
Most soils have tens to hundreds of mineral species comprising their solid fabric. Those minerals commonly fall into three common groups: (a) silicates, (b) carbonates and (c) oxides/hydroxides. It is important to note that soil regions in the world also contain significant sulfate, halide and/or phosphate minerals; however, this chapter focuses on the silicates, carbonates and oxides/hydroxides. Two important subgroups within the silicates are the tectosilicates (3D lattice framework of Si-tetrahedrons) and phyllosilicates (2D framework of aluminosilicates comprised of Si-tetrahedral layers bonded to Al- or Mg-octahedral layers – e.g., see Nelson. 2015., Gaston. 2015).
Learn more about soil minerals, their composition, and main functional importance: Soil minerals[1]
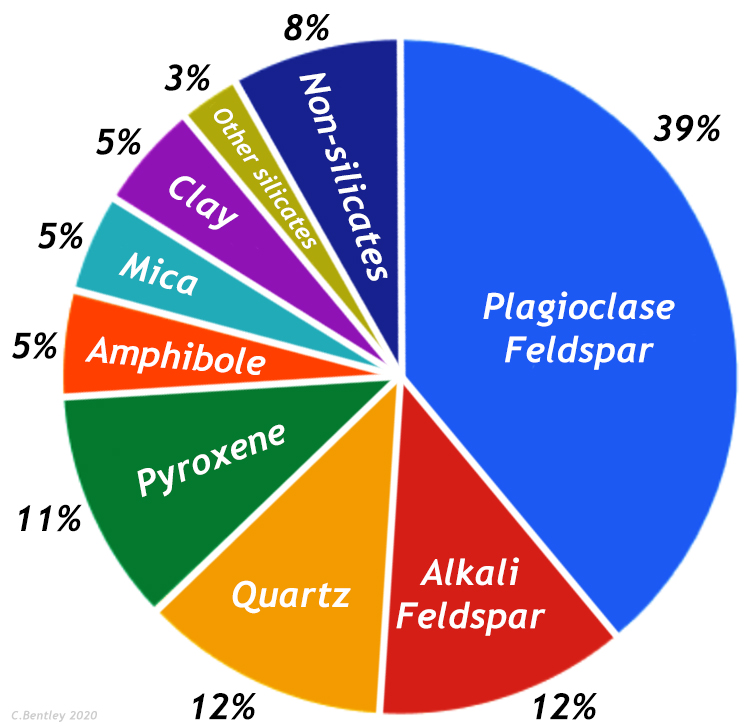
Importance of soil minerals
The importance of a mineral in soil is dependent on its prevalence and/or its degree of reactivity. Prevalence matters because minerals they form the soil’s physical framework, accounting for 99.9% of the dry mass in most B and C horizons. In other words, it is the mineral fraction that controls a soil’s solid phase characteristics A mineral’s reactivity – aka, dynamism – is controlled by its chemical composition, kinetic reactivity, solubility, and/or charge.
There are three pathways whereby a mineral is present in a soil. The most common is inheritance from the parent material. As a result, whatever mechanism that caused the glacial till, loess, volcanic ash, alluvium, colluvium, lava flow – whatever – to be located in a location is what brought or created the original minerals in that soil. The second pathway is some kind of subsequent physical addition. Two common examples of this are aeolian dust and human additions, e.g., ag lime or some fertilizer including fillers within the fertilizer. The third pathway is pedogenesis during which time an inherited or added mineral dynamically changes form.
The dynamic nature of minerals in soils takes many forms. It can be as simple as hydration – e.g., anhydrite (CaSO4) sorbs (loosely bonds) water molecules onto its surface and creates gypsum (CaSO4*nH20). Hydrolysis is another simple process involving water – e.g., quicklime (CaO) sorbs water molecules, which then split, and the resulting mineral is portlandite (Ca(OH)2). In this process the soil solution gains two H+, which will at least temporarily acidify the soil solution and likely initiate the weathering of another mineral. Hydration and hydrolysis of feldspars are important steps in the conversion of feldspars into phyllosilicates. More broadly, hydration and hydrolysis of rocks and stones coupled with wetting and drying and/or heating and colling results in large soil solids (think boulders, cobbles and gravel) disintegrating into sand or even silt and clay. Oxidation-reduction reactions are likewise simple reactions that routinely dissolve, form or do both with iron- and manganese-bearing minerals. Carbonation drives change in many minerals whenever carbon dioxide (CO2) is introduced in the soil solution. An astute reader will immediately realize that plant and microbial life is constantly added CO2 throughout the root zone with the result always being mineral weathering.
Mineral weathering and CEC
A more commonly discussed example of mineral weathering is that of the phyllosilicates, a.k.a., “clay minerals. Soil weathering of illite routinely causes the interlayer potassium (K+) to pop out of its atomic lattice and move into soil solution, where it is routinely uptaken by a plant root. The resulting illite crystal has a slight shift in its tetrahedral basal oxygens and has its layer charge from Al-substitution turn immediately into CEC. Without going into huge detail that simple loss of K+ is the cause of the incredible differences between illite and vermiculite vis-à-vis surface area, CEC and shrink-swell. The vermiculite crystal produced as a result of K+ removal from illite can be highly stable, or, – depending on the distribution of Al-substitution in the Si-tetrahedrons – can rapidly break into smaller pieces where some of the A3+ escapes the atomic lattice. When that happens smectite forms (Burras, 1992). That smectite has huge physical and chemical differences from its vermiculite parent, just as the vermiculite parent differs from its illite parent. Additionally, the smectite being a very small crystal is able to lessivage deeper into the soil profile; thereby, contributing to the formation of an argillic horizon. Alternatively, if the smectite stays in place it can contribute to vertic horizon characteristics.
Returning to the vermiculite, if its layer charge is remains high (e.g., no escape of Al3+ at its crystal edges) then the addition of K+ fertilizers can result in “K-fixation” and the recreation of an Illite crystal. Importantly, if ammonium (NH4+) fertilizer is added to that high charge vermiculite then “ammonium fixation” can occur, resulting in a pseudomorph of Illite. Either form of fixation results in less fertilizer nutrient being available than otherwise expected.
To sum the previous paragraphs up, minerals change in soil. They change due to natural processes. They change due to human impacts. Some changes are physical movement. Some changes are chemically driven. Some changes are congruent, which means the complete dissolution of the original mineral. Some changes incongruent, which means the original crystal only partially dissolves, but as a result it turns into a new mineral. In summary, soils are predominately composed of minerals with every one of those minerals prone to change as the soil forms and/or is used by humans. With enough time those changes will drive an incredibly fertile Entisol to become a challenging Ultisol or even an Oxisol.
- Soils are mostly minerals.
- In the A horizon, soil mineralogy influences root growth through nutrient dynamics and available water dynamics.
- In the B and C horizons, soil mineralogy controls soil physical and chemical properties.
- Clay mineralogy is especially reactive although all minerals in soil change at least slowly.
References
Allen, B.L. and D.S. Fanning. 1983. Composition and Soil Genesis. Chapter 6. In: L.P. Wilding, N.E. Smeck and G.F. Hall (Editors). Pedogenesis and Soil Taxonomy. Volume 1: Concepts and Interactions. Elsivier, Amsterdam. p. 141-193.
Burras, C.L. 1992. Origin of Smectite in Soils of Western Ohio. PhD Dissertation, 334 p. The Ohio State University Library. Available at: https://etd.ohiolink.edu/acprod/odb_etd/etd/r/1501/10?clear=10&p10_accession_num=osu1487777901658994 , reviewed December 07, 2023.
Gaston, L. 2015. Soil Colloids. Agro/EMS 2051. Soil Science. Available at: http://www.agronomy.lsu.edu/courses/agro2051/chap08.htm , reviewed December 07, 2023.
Nelson, S.A. 2015. Phyllosilicates (Micas, Chlorite, Talc, & Serpentine). EENS 2110. Mineralogy. Available at https://www2.tulane.edu/~sanelson/eens211/phyllosilicates.htm , reviewed December 07, 2023.
- https://iastate.pressbooks.pub/app/uploads/sites/68/2022/02/Soil-minerals-Burras.pdf ↵

