Soil pH
Ala Khaleel and Amber Anderson
- Identify pH values associated with basic or acidic conditions
- Identify basic vs. acid-forming cations
- Discuss the impact of pH on soil and plant growth
Soil pH
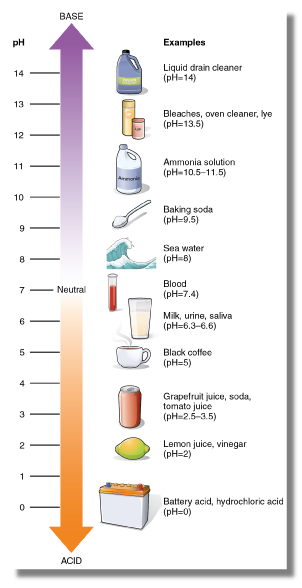
Soil pH is the measure of soil acidity or alkalinity, specifically the inverse log of the Hydrogen ion concentration on a scale from 0-14. Neutral pH is around 7, with ‘acids’ being below 7 and ‘bases’ being from 7 to 14. Therefore, a change from a pH value of 5 to a pH value of 4 indicates a 10x increase in H+. Most soils have pH values between 4 to 10. Most soils in Iowa have a pH between 5.5 to 7.5. More weathered soils generally have lower pH values, with soils in arid regions having higher pH values due to accumulations of calcium or sodium.
Some soils have higher ‘buffering capacity’ or ability to resist change. In higher organic matter or those high in particular types of clays, those with higher CEC values, the same management will have a lesser impact on the pH. Therefore, when trying to raise the pH, additional lime or input will be required.
Consider a perfectly hot large coffee pot vs. a cup freshly poured in your hand. Predict which would change temperature most quickly if an ice cube were added.
The larger volume of the coffee pot makes the temperature change more slowly than your cup and has a higher ability to resist that temperature change. This would be the higher buffering capacity in the soils example. Small changes in either direction-either an ice cube added or perhaps 30 seconds in the microwave would be expected to have a bigger change on the temperature of your cup than of the large coffee pot.
Importance
Soil pH is sometimes considered the “master variable” that has several impacts on plant nutrients and plant growth. Soil pH also impacts or interacts with other properties in the soil.
Soil pH influences
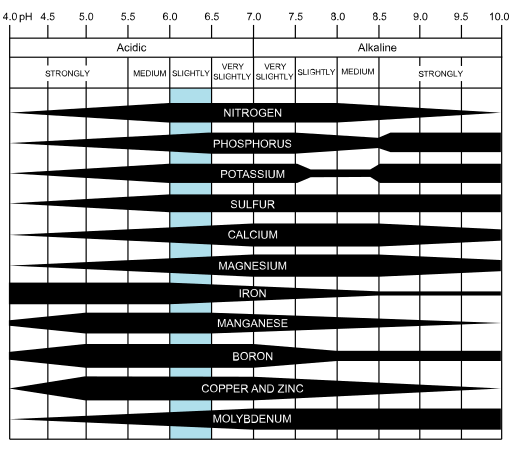
- Amount and availability of plant nutrients; some plant nutrients are more available under acidic conditions, while others are more available under basic or alkaline conditions
- The activity of soil microorganisms responsible for residue decomposition
- Charges on soil organic matter and on some mineral surfaces, influencing the soil’s cation exchange capacity
Factors influencing pH values in soil
- Parent material: original pH of the material.
- Nitrogen fertilization: over time, can lower pH values.
- Management for particular crop: agricultural lime or ‘liming materials’ may be added to raise pH, or elemental sulfur or acidifying materials may be added to lower the pH for a particular target crop.
- Time: over time, removal of base-forming cations with weathering or cultivation will decrease the soil pH
- Buffering capacity: the soil’s ability to resist change or a higher buffering capacity, means that a soil pH will be slower to change with the same action that may more significantly lower the value elsewhere.

The color of old-fashioned hydrangeas are an indicator of pH, displaying blue flowers in acidic conditions and pink in higher pH-alkaline soils.
This hydrangea was photographed in the highland regions in Costa Rica:
Raising the pH
For the lime requirement guidelines, especially for Iowa or the Midwest US, you can consult the Iowa State University soil pH and lime application guidelines (extension publication PM 1688).
- In the lab, the pH of a soil solution is usually measured with a glass electrode. The soil sample is prepared with either water or dilute salt solution, as the electrode only measures acidity in solution rather than H+ ions held on the soil surface.
- In the field, pH paper strips can be used to get an approximate value to determine the need for further testing or management.
- Soil pH is an important factor in the soil, influencing nutrient availability and organism activity
- pH value of soil is impacted by management, such as crop removal or limining
- pH value can be increased by application of liming materials, or decreased by sulfur-containing materials

