Common Soil Phyllosilicates
Rivka Fidel
Upon completing this chapter, you should be able to:
- Match phyllosilicate properties with simple diagrams representing each phyllosilicate*
- Explain why and how phyllosilicates in soil vary with respect to degree of soil weathering
*at the University of Arizona and Iowa State University, knowing the details of phyllosilicates is optional for introductory soil science. Elsewhere, please check your course website or ask your instructor.
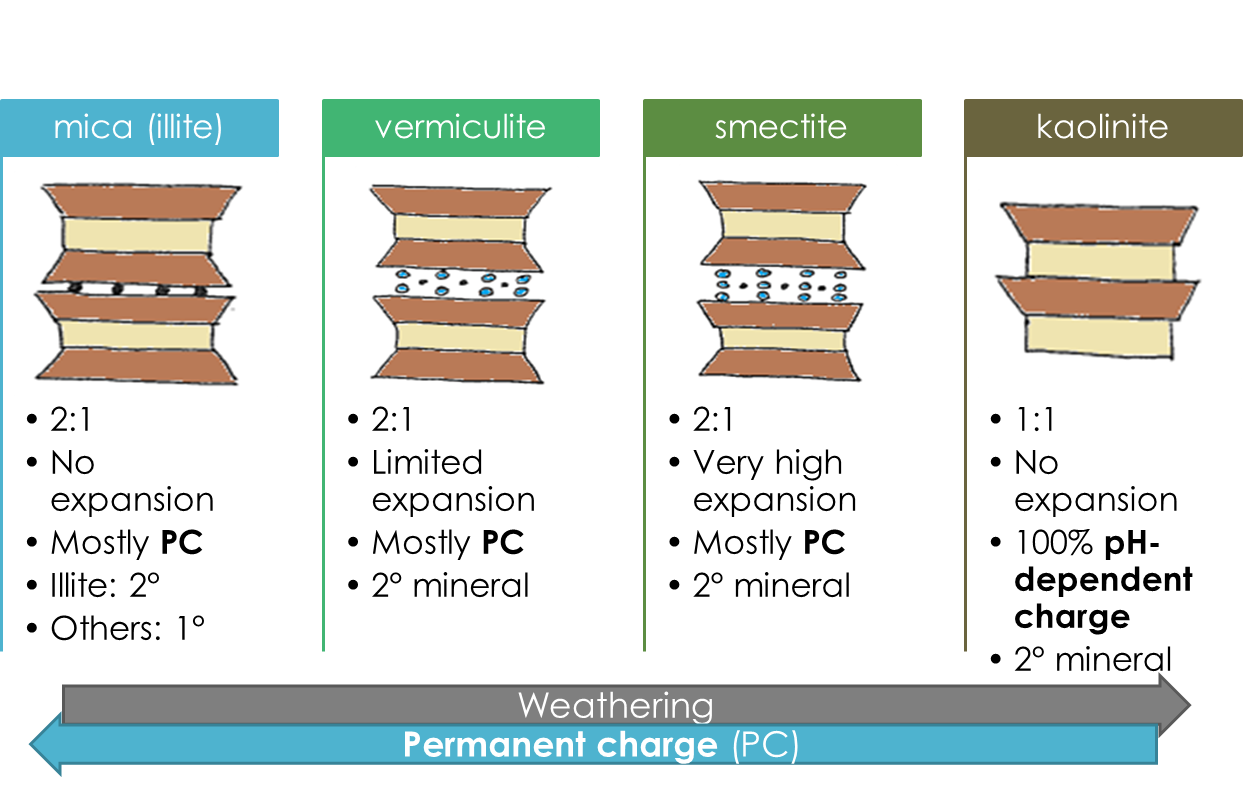
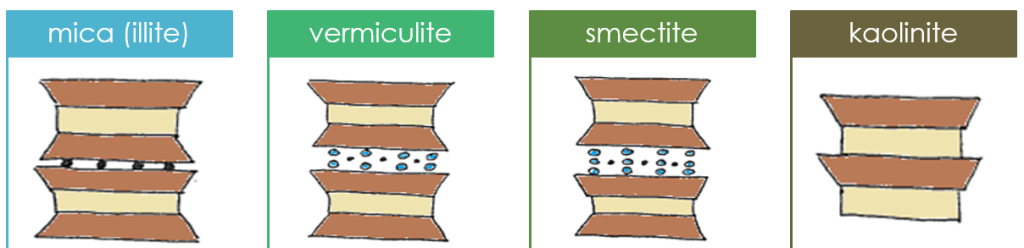
Phyllosilicates are classified based on their layer composition, layering pattern (2:1 vs 1:1), and interlayer composition – which influence their charges and hence ability to hold onto nutrient and/or contaminant ions. Interlayer composition also influences the ability of the mineral to swell – a phenomenon where water enters and expands the interlayer. The four most common phyllosilicates in soil, in order of increasing degree of weathering, are mica (frequently as illite), vermiculite, smectite, and kaolinite.
Mica (Illite)
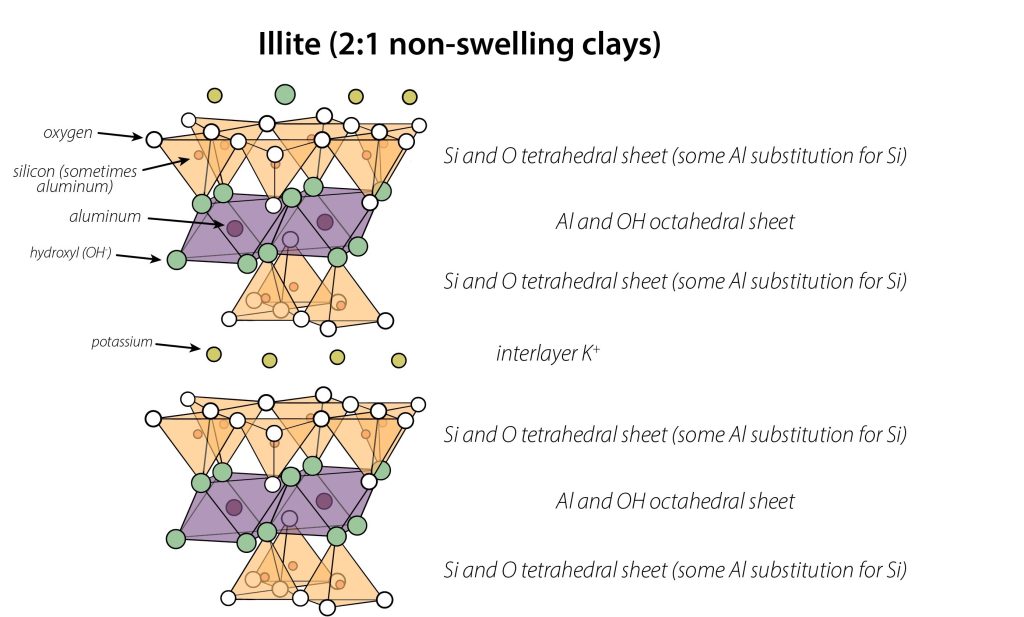
Mica is the least weathered phyllosilicate, a primary mineral (i.e. formed from the cooling of magma), characterized by a 2:1 layering pattern. It undergoes isomorphous substitution in the tetrahedral sheet during formation, leading to copious permanent charges. The interlayer of mica contains K+, but no water. This K+ fits perfectly into little gaps on the interlayer-facing surface of mica, which encourages the interlayer to clamp closed around the K+. You can think of it like an egg carton – the eggs fit perfectly in egg-shaped divots found in the carton, enabling the carton to close. This tight fit of mica layers around K+ interlayers does not allow water to enter, so mica does not swell. This limits mica’s surface area, limiting its ability to retain ions despite its high permanent charge.
 |
 |
You may have seen microscopic mica particles before, as they frequently lend a slight shimmer to sediments in which they’re found. This shimmering property, called luster, makes mica a great mineral glitter used in “natural” makeup and nail polish. It is also used as an additive in other products, for example to improve the properties of drywall, asphalt shingles, and even some electronics.
In soil, properties of mica are more variable, and the mica particles are typically smaller due to weathering – so you can’t spot them with your naked eye. Such “soil mica” particles typically have fewer layers per particle, and their exact chemical formulas vary from soil to soil. This clay-sized “soil mica” is called illite. In this introductory text, we treat mica and illite as synonymous, whereas differences between the two become important in advanced soil science texts.
 |
 |
 |
 |
Vermiculite
Vermiculite forms from the weathering of mica (and chlorite, see chlorite box), and is characterized by a 2:1 layering pattern with mostly Mg2+ , Ca+2, and water in the interlayer. Typically about 1/7th – 1/4th of tetrahedron in the tetrahedral sheets of vermiculite have experienced isomorphous substation where Al3+ has replaced Si4+. In soil, Mg2+ and even Mn2+ may also substitute for a smaller proportion Al3+ in the octahedral sheet (perhaps 1/10th-1/8th of octahedra). This leads to a high permanent charge, but not as much as mica.
Unlike K+ in mica, the Mg2+ and Ca+2 found in vermiculite interlayers do not fit into the gaps of the interlayer crystal surface (called “ditrigonal holes”). Instead, Mg2+ and Ca+2 have smaller ionic radii, and so they “rattle around” in the interlayer. Without a snug fit, they allow water to enter, and the interlayer swells. In sufficiently moist conditions, vermiculite interlayers can have up to two planes of water molecules. This limited expansion leads to a moderate degree of shrink-swell behavior in vermiculites.
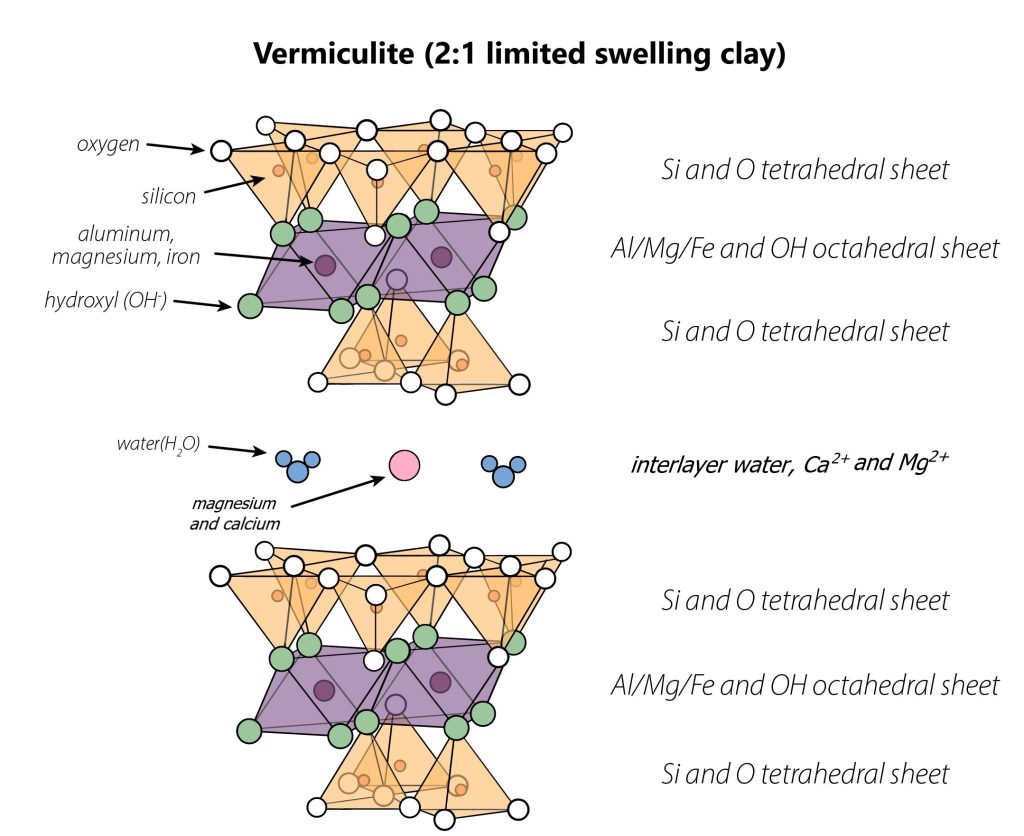
Vermiculite is used for insulation in homes as well as in potting media. In insulation, the layers are forcibly expanded with air instead of water to create insulating air pockets like in a down jacket. In potting media, vermiculite enhances retention of water and ions.
 |
 |
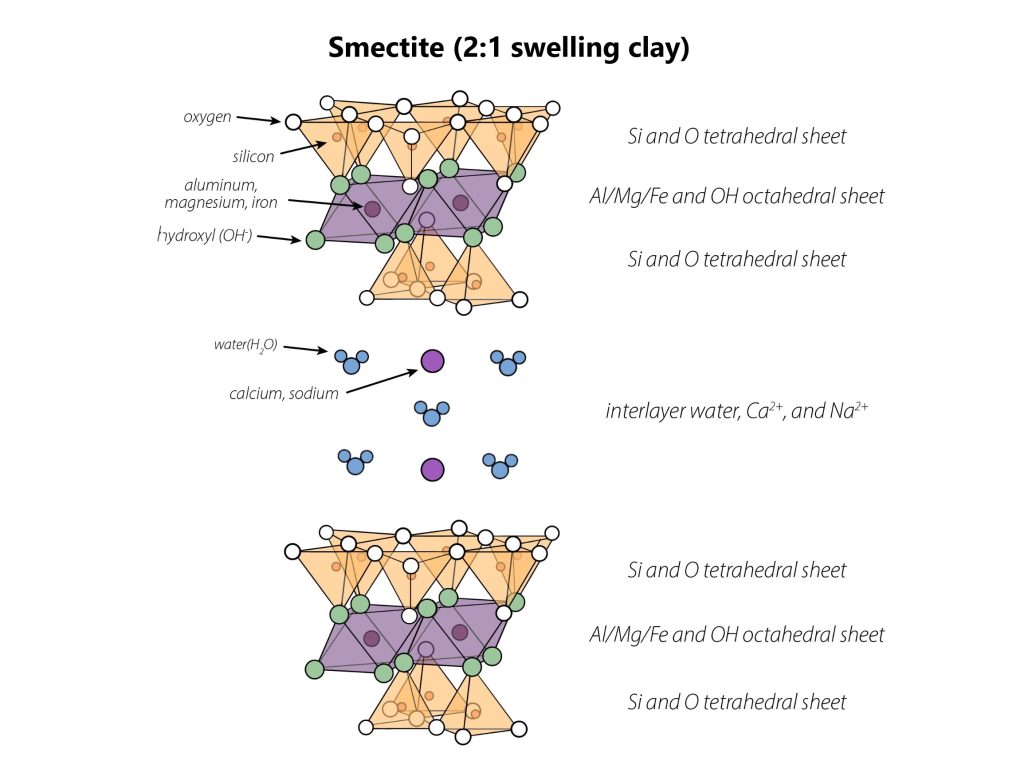
Smectite
Smectite forms from weathering of mica and vermiculite, and inherits a 2:1 layering pattern from these minerals. Unlike its mineral “parents”, smectite exhibits isomorphous substitution primarily in its octahedral sheet, where Mg2+ substitutes for Al3+. Since octahedral sheets are further from the interlayer, negative charge at the interlayer isn’t “felt” as strongly so cations aren’t held as strongly in the interlayer. Smectite also has Ca2+ in its interlayer, and some smectites in sodium-rich soils and sediments also contain Na+ in their interlayers. Together with the more distant and diffuse octahedral charge of smectite, these Ca2+ and Na+ ions allows for the greatest degree of swelling among the common phyllosilicates: up to 5 layers of water may be present in each interlayer.
Smectites are responsible for the large degree of swelling seen in Vertisols, and other smectite-rich soils likewise exhibit greater shrink-swell behavior than soils lacking in smectites. A common type of soil smectite is called montmorillonite. In sediments and sedimentary rocks, montmorillonite is found in bentonite, which is a smectite-rich sediment primarily composed of montmorillonite (in Geology, bentonite refers to sedimentary rock – a claystone – formed from montmorillonite-rich sediment). Bentonite is used to make products like kitty litter because it is water-absorbent and sticky, allowing it to clump together when wet as well. Because smectite particles are very small, they exhibit very slow water permeability, and therefore bentonite is used to line water sampling wells, as well as waste storage lagoons.
Kaolinite
Kaolinite has a 1:1 layering pattern, and is the most weathered of the common phyllosilicates. It can form from any other phyllosilicate; for example mica frequently weathers into vermiculite, then smectite, then kaolinite.
Kaolinite’s 1:1 layering pattern means there is no interlayer, and hence no opportunity for water to enter or for the clay to swell. Due to its lack of interlayer, kaolinite also

has the lowest surface area of the common phyllosilicates. Additionally, kaolinite has no permanent charge. So, all of its charge originates from broken edges, and is hence pH-dependent.

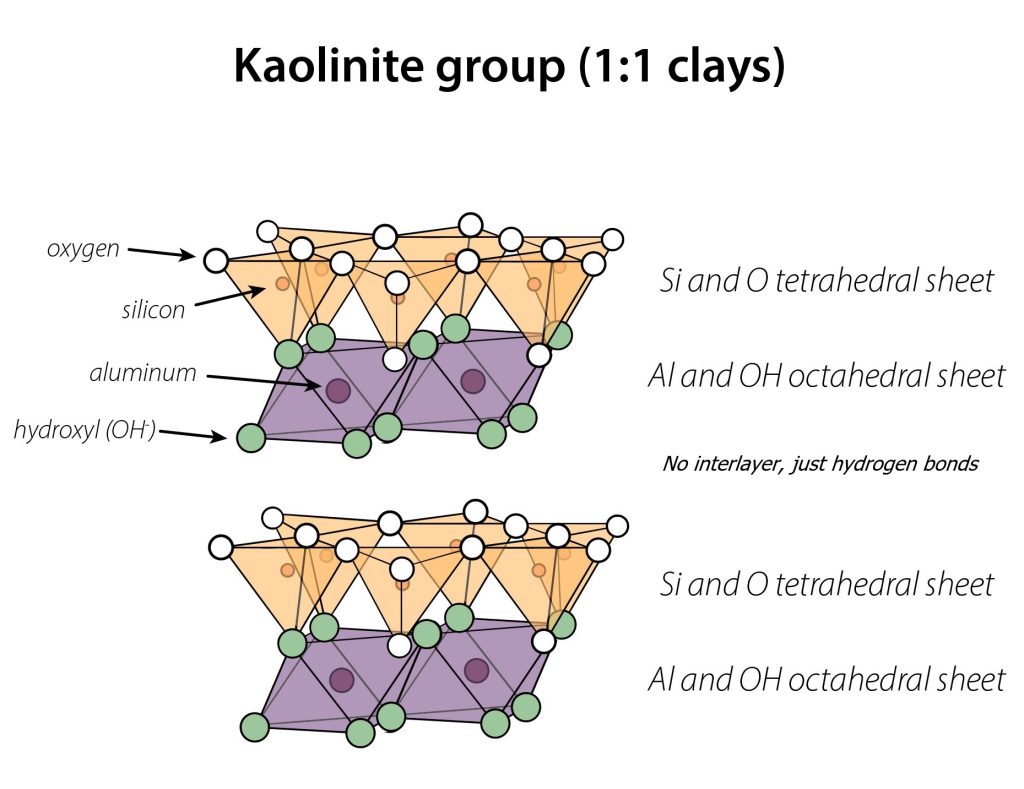
The lack of swelling behavior of kaolinite makes it useful for making pottery and infrastructure. Use of kaolinite in pottery and porcelain prevents the cracking and breakage that occurs during drying and/or firing clay when too much swelling clay is included in the potting clay mix. Using kaolinite (typically mixed with sand) underneath roads and buildings also helps avoid the kind of swelling that would cause the ground to rise and fall underneath, resulting in road/building damage.
- Cookies = tetrahedral sheets
- Creme = octahedral sheets
- Spaces between cookies = interlayers with water
- Peanut butter = interlayer with potassium ions only
| Mica (2:1) | Vermiculite (2:1) | Smectite (2:1) |
Kaolinite (1:1) |

|
 |

|
 |
|
Peanut butter represents K+ in the interlayer. |
The limited space between cookies represents limited expansion in the interlayer. | The large space between cookies represents a large degree of expansion in the interlayer. | Note how there’s cream in between each cookie, representing a TO-TO, or 1:1 pattern. There are no gaps, because kaolinite has no interlayer. |
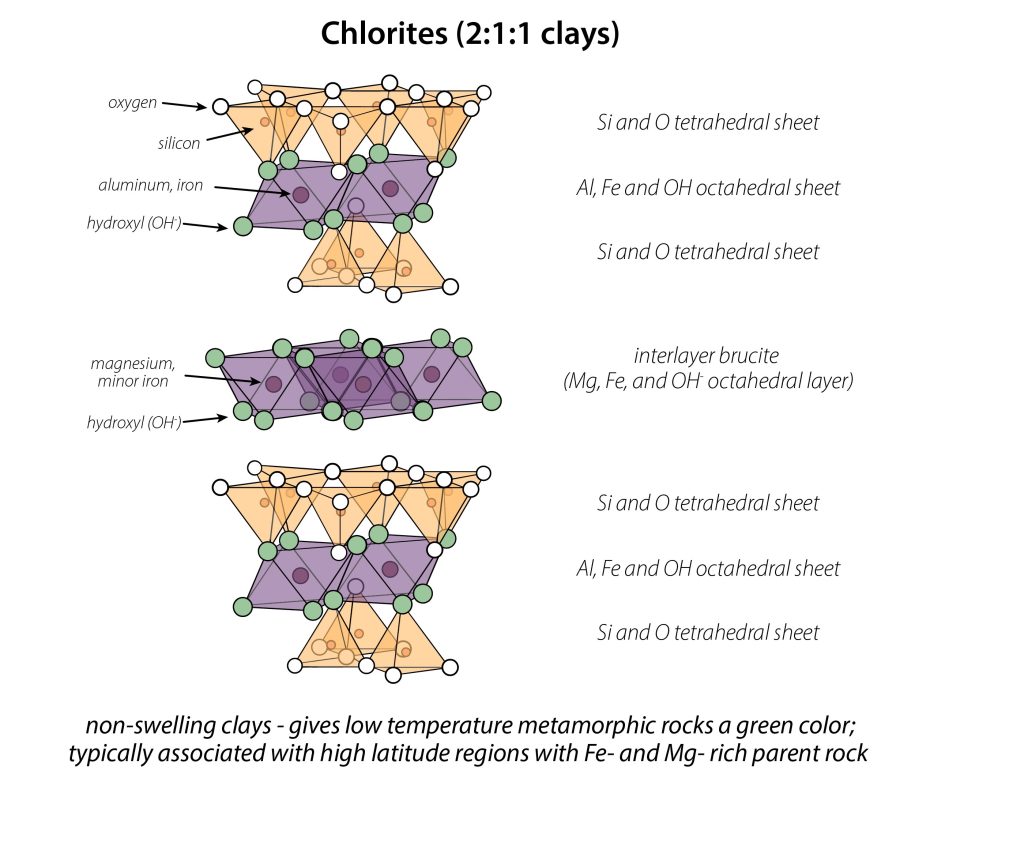
Chlorite, a 2:1:1 Phyllosilicate
At the introductory level, we focus on the most common clays in soil, which have 2:1 and 1:1 layering patterns. However, there is a 3rd layering pattern, called 2:1:1. These phyllosilicates, in addition to tetrahedral and octahedral sheets that are bonded to each other, have an octahedral sheet forming in the interlayer. Although at first glance the layers alternate tetrahedral-octahedral, the 2:1:1 layering pattern is unique since the extra interlayer octahedral sheet is distinct in composition from the one found sandwiched between tetrahedral sheets within the layer. This extra interlayer sheet prevents the phyllosilicate from collapsing and expanding, so 2:1:1 clays cannot swell. The most common of these 2:1:1 phyllosilicates is chlorite, shown below.
Trends among the four main phyllosilicates’ properties
You may have noticed some general trends among phyllosilicate properties. With increasing weathering – going in the order mica vermiculite smectite kaolinite – the following trends can be observed:
- Decreasing permanent charge
- Increasing and then decreasing expansion and swelling
- Increasing and then decreasing surface area
- Increasing and then decreasing ion retention
Learn more about ion retention of phyllosilicates and other colloids in upcoming chapters.
You can examine interactive 3D clay structures online at the Virtual Museum of Minerals and Molecules! Here are direct links to each phyllosilicates from this chapter:
- mica: muscovite and biotite
- vermiculite
- smectite as found in soil based on research by Dr. David Laird (1991), water omitted
- kaolinite
Note that the default view is not the same as what’s shown in the figures of this chapter. Use your mouse to rotate the structures until they appear familiar. There are also options to highlight specific elements or parts of each structure.