Importance of Soil Chemical Properties
Rivka Fidel
- Define: pH, EC, solution
- Explain the importance of soil chemical properties and surfaces
- Sort the solution, surfaces, and solids in order of relative chemical reaction rates
Why are soil chemical properties important?
Soil chemical properties have widespread impacts on countless soil processes, and therefore their importance cannot be overstated. The chemicals in soil, from solid minerals to nutrients and other salts in solution, affect not only plant growth and microbial diversity but also soil greenhouse gas emissions and the transport and breakdown rate of nearly any chemical from nutrients to hazardous substances. Therefore, understanding soil chemistry is vital to understanding and managing soil.
Chemicals are found everywhere in the soil – in fact, soil is made of chemicals. As such, soil contains countless chemicals, reacting with each other constantly. The enormous diversity of both chemicals and their reactions in soil can become quite overwhelming but becomes much more understandable when we organize them according to phase: solids, liquids (here, aqueous solutions), and gases. Of particular importance in this course are the solids and liquids, and their interactions at the solid-solution interface.
Soil Solids
You may recall that an average soil is about 50% solid particles, where some of these are mineral and some are organic. In soil science, minerals are inorganic, meaning they do not contain carbon (with the major exception of carbonates). Organic particles on the other hand, do contain carbon – specifically carbon atoms bonded to other carbon atoms, or to hydrogen atoms (thus, carbonates are excluded, as CO32- has only C-O bonds).
Minerals are important not only for providing nutrients as they weather and dissolve, but also for providing structure to the soil and surfaces for other chemicals to adhere or “adsorb” to (more on this later). Some minerals weather faster, whereas others resist weathering, and as such their chemical properties affect the overall rate of soil formation.
Organic particles release nutrients into the solution as they break down during the decomposition process (distinct from weathering, see Soil Life or Soil Organic Matter chapter). Organic matter also has a very high surface area and water holding capacity, helping it to hold onto water and nutrients.
Both mineral and organic particles are found in a wide range of sizes, from >2 mm down to less than 1 micron (that’s 0.001 mm). Different size fractions of these particles behave differently in soil, especially in the way they affect the soil solution.
The Soil Solution
The soil solution is all liquid water within the soil and the solutes dissolved within. Solutes include ions, as well as neutrally charged species like sugars and select acids and bases. Gases such as carbon dioxide (CO2) and oxygen (O2) can also dissolve into the solution and react with other solutes, as well as water. During the dissolution process, each molecule of solute is surrounded by water molecules, affecting the behavior of the overall solution.
Review: what is a solution?
A solution is a homogenous mixture that is made when a solid or gas has dissolved into a liquid. During dissolution, liquid molecules completely surround each molecule of solid (or gas). For example, when table salt (NaCl) dissolves, each Na+ ion is fully surrounded by a sphere of water, and so is each Cl–.
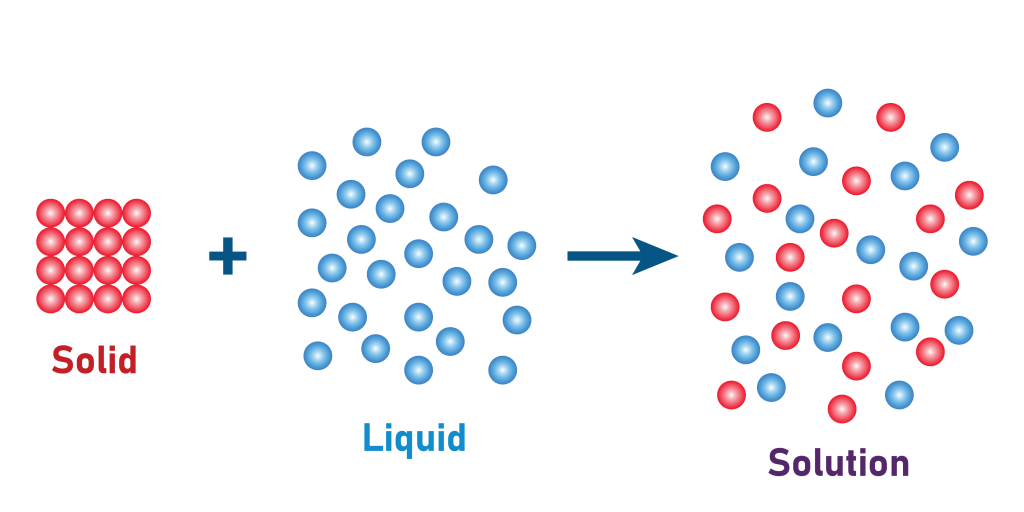
In soil, solid mineral salts like CaCO3, CaSO4 and NaCl are relatively soluble and therefore dissolve readily, becoming part of the soil solution in most soils (albeit at widely varying concentrations depending on the soil’s mineral composition and formation factors). Greenhouse gases like carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), as products of microbial metabolism, are nearly ubiquitous in soils.
Because solids react very slowly compared to solutes and liquids, and because water is required for most reactions, the solution is where the majority of soil reactions occur. Thus, soil solution properties dramatically affect soil reactions.
Three very important soil solution properties that have cascading effects on soil behavior are pH, redox status, and electrical conductivity. We’ll discuss these further in other chapters, but here is a brief overview for now:
- Soil pH, an measure of soil acidity and basicity that is calculated as the negative log of the hydrogen ion concentration: pH = -log[H]
- Redox status, as measured by redox potential (Eh), [O2], or [CO2].
- Redox reactions are greatly influenced by the amount of oxygen and other oxidizing agents in soil such as nitrate.
- Soil redox potential (Eh) is a measure of the electrical potential of soil due to the tendency of the chemicals within it to donate or accept electrons. The more oxygen present, for example, the more important nutrients like nitrogen and iron can become oxidized by donating an electron to oxygen.
- Electrical conductivity (EC), a measure of how easily electrons can flow through soil.
- EC is directly proportional to the total concentration of ions in solution, and thus EC is a good measure of salinity or soil “saltiness.” Salinity affects numerous soil properties such as aggregation, infiltration, and drainage. It also affects soil osmotic potential and therefore water uptake by plants.
- The total ion concentration in soil also affects reaction rates, but that is beyond the scope of this class. See [LINK].
Soil Surfaces: The Solid-Solution Interface
Due to the very slow reaction rates of solids, the vast majority of reactions with solids occur at the solid-solution interface, aka the surfaces of soil particles where they meet soil water. These reactions include dissolution of the solids into the solution, precipitation of new solids onto the solution, and adsorption of solutes onto surfaces. (This one of the reasons why particle size and surface area are so important in soil science – see below review box for more details).
You can picture adsorption as the “sticking” or adherence of solutes to solid particle surfaces. Nutrients and contaminants alike can both adsorb to soil particle surfaces, enabling soil to retain nutrients for plants while keeping contaminants out of the groundwater. Thus, soil surfaces support three important soil ecosystem services: supporting plant growth, providing nutrients, and filtering water. We will learn how this works in upcoming chapters.
Review: Surface Area & Particle Size Relationships

- Soil chemical properties affect the soil’s ability to provide important ecosystem services such as supporting plant growth and filtering water.
- Important soil chemicals are found in solids, the solution, and on soil surfaces at the solid-solution interface.
- Of these, the solution and surfaces are much more reactive than inner portions of solid particles.
- pH, redox status, and EC are all very important soil solution properties that each affect numerous soil behaviors.
- Soil surfaces are unique in enabling adsorption, the process by which soil retains nutrients and contaminants. This keeps nutrients in the soil where plants need them while keeping contaminants out of groundwater.

