Poorly crystalline silicate, oxide, and organic colloids
Rivka Fidel
After completing this chapter, you should be able to:
- Compare and contrast types of colloids in terms of:
- General composition
- Type or origin of surface charge
- Where you would expect to find them in abundance
Review of Colloid Types
Recall from the previous chapters that there are 4 types of colloids in soil:
- Crystalline silicate clays (phyllosilicates): layered minerals containing SiO4
- Poorly crystalline silicates: sphere- and tube-shaped minerals containing SiO4
- Oxides and hydroxides: highly weathered minerals containing O2-, OH–, and OOH3-, bonded to transition and post-transition metals (mainly Fe and Al)
- Organic colloids: particles of biological origin <1 μm in size that contain C-H and/or C-C bonds, also called “colloidal humus”
Previously, we discussed the crystalline silicate clays, also known as phyllosilicates or “layer clays”. Here we will discuss the remaining 3 types of colloids.
Poorly crystalline silicates
Poorly crystalline silicates are SiO4-based minerals containing what is called “short-range order”, meaning that the patterns in which their elements are arranged only repeat a few times instead of thousands of times or more. They tend to have sphere- or tube-shaped structures, and form during weathering of volcanic ash. Volcanic ash is unique in that it contains very small pieces of volcanic glass, so non-crystalline SiO4, instead of more common minerals like quartz, feldspar, and mica. Glass, or “glassy silica” lacks crystallinity because it forms from very rapid to instantaneous cooling of lava. It is from this non-crystalline, glassy precursor that the poorly crystalline silicates get their lack of crystallinity. Indeed, some soil scientists and minerologists consider the crystallinity of these minerals to be so poor that they call them “non-crystalline silicates” instead, grouping them with glass.
Two of the most common poorly crystalline silicates are allophane and imogolite. Their sphere and tube shapes help them retain more plant-available water than phyllosilicates and oxides or hydroxides (the latter is discussed later in this chapter). This water retention greatly improves the fertility of Andosols (volcanic ash soils). Additionally, allophane and imogolite have substantial pH-dependent charge, helping them hold onto cations and/or anions, depending on the pH.

Oxides and Hydroxides
Oxide and hydroxide minerals are the most weathered of the mineral colloids, and are therefore most concentrated in highly weathered soils like Ultisols and Oxisols. They can be crystalline, poorly crystalline, or non-crystalline. Oxide and hydroxide minerals are named after their anions, oxide, O2-, and hydroxide, OH–. When oxide and hydroxide are together, they form oxyhydroxide, OOH–. For simplicity, we refer to oxyhydroxide minerals as a type of oxide mineral, since they contain oxide. In oxide and hydroxide minerals found in soil, the negative charges of these ions are balanced by either Al3+, a post-transition metal, or by common transition metal cations like Fe3+, Mn4+, Cu2+, and Zn2+. Since Fe and Al are far more abundant in most soils than other transition and post-transition metals, will focus here on Fe and Al oxides and hydroxides.
Iron oxides and hydroxides
Iron oxide and hydroxide minerals are quite diverse in both structure and color. Iron (III) oxide minerals range from goethite (FeOOH), which lends many temperate soils their yellow-to-brown hues, to hematite (Fe2O3), which brings a red color to Ultisols and Oxisols. Iron (II) hydroxides like Fe(OH)2 are found in saturated soils, given that Fe2+ normally oxidizes to Fe3+ when oxygen is present. These “green rusts” are blue to green in color, and are common in wetland subsoils and clay inclusions in other soils. Iron oxides and hydroxides, like most oxides and hydroxides, exhibit very poor nutrient retention. However, iron specifically can bond strongly with phosphate, keeping this water pollutant and nutrient in the soil.


Aluminum hydroxides
Unlike iron, aluminum minerals lack color. In soil, aluminum oxides such as corundum (Al2O3) are rare, because they readily weather into aluminum hydroxides, and therefore aluminum hydroxides dominate. The most common of these is gibbsite, Al(OH)3. Gibbsite has stacks of octahedral sheets containing a plane of Al in the center, like in phyllosilicates. Each layer contains about 6 octahedral sheets. However, gibbsite has only aluminum, oxygen, and hydrogen – no silicon. So, it is not a silicate. Aluminum in gibbsite does not provide nutrition to plants (it’s toxic), but gibbsite can retain a small amount of nutrients – much smaller than other colloids. This is one of the reasons that highly weathered soils, often rich in gibbsite, are not fertile.
This table shows names, formulas, colors and other information about common oxide and hydroxide minerals found in soil. (Adapted from NRCS “The Color of Soil” https://www.nrcs.usda.gov/sites/default/files/2022-11/color-of-soil.pdf with the addition of gibbsite and magnetite)
|
Mineral |
Formula |
Munsell Color Info |
Common Color |
|
gibbsite |
Al(OH)3 |
Any hue, value of 4 to 7 and chroma less than 2 |
grey |
|
goethite |
FeOOH |
10YR 8/6 to 7.5YR 5/6 |
Yellow to brown |
|
hematite |
Fe2O3 |
5R 3/6 to 10R 4/8 |
red |
|
lepidocrocite |
FeOOH |
5YR 6/8 to 2.5YR 4/6 |
reddish-orange |
|
ferrihydrite |
Fe (OH)3 |
2.5YR 3/6 |
orange |
|
magnetite |
Fe3O4 |
Value of 2 or less |
black |
|
maghemite |
– Fe2O3 |
Hue: 2.5YR-5YR |
orange-brown |
|
todorokite |
MnO4 |
10YR 2/1 |
black |
Surface charges of oxides and hydroxides
Oxides and hydroxides generally have small amounts of pH-dependent charge, and little to no permanent charge. This is because their charged functional groups – mainly hydroxyls, aka OH’s – are located primarily on the surface, where they can easily accept or donate H+ from the solution.
You can view interactive 3D structures of various oxide and hydroxide minerals by clicking these links:
Organic Colloids
Organic colloids are particles <1 μm in size derived from living organisms that contain primarily C, H, and O. Like all organic matter, these tiny organic matter particles are distinct from carbonate (CO32-) minerals in that they contain C-C and C-H bonds. Typically, these small particles form during decomposition of larger organic particles such as pieces of leaves, stems, or roots. Some also form from dead microbial cells. Because of relatively large extent of decomposition required to break organic particles down to <1 μm, generally organic colloids are sufficiently decomposed to be considered a part of humus (humus can be any particle size, though, since it’s defined a highly decomposed, amorphous organic matter).
A closer look at organic matter particles
Organic matter particles come in many shapes and sizes. They cannot be viewed with a traditional light microscope since they block light, and so are frequently imaged with a Scanning Electron Microscope (SEM) instead. Here’s an example image that includes a wide range of organic matter particle sizes (only the very smallest are colloids):

Because organic colloids are a type of organic matter, they are most concentrated in the O and A horizons of many soils. Organic colloids would also be especially abundant in Histosols, given the very large O-horizons found in these wetland soils.
Organic colloids are important for similar reasons that organic matter and colloids in general are important. The area where they really stand out its their ability to hold onto nutrients and filter contaminants. Organic colloids have copious pH-dependent charge sites composed of organic functional groups like carboxylic acids. This lends them a very high cation exchange capacity at neutral to alkaline pH’s, which we’ll discuss in the next two chapters.
One of the most common surface functional groups in organic colloids is carboxylic acid. As pH increases, carboxylic acid donates H+ to the solution, and develops a negative charge.
In the diagram below, “R” is any functional group in an organic matter particle. Carboxylic acid functional groups, as shown in the middle of the figure, have the formula -COOH. Two oxygens are bonded to a central C, and one of the O’s is double bonded to the C. The other O is bonded to H. Usually, carboxylic acids only have one H, but in organic matter particles, it’s theorized that very large R groups may stabilize the carboxylic acid enough that it could accept another proton.
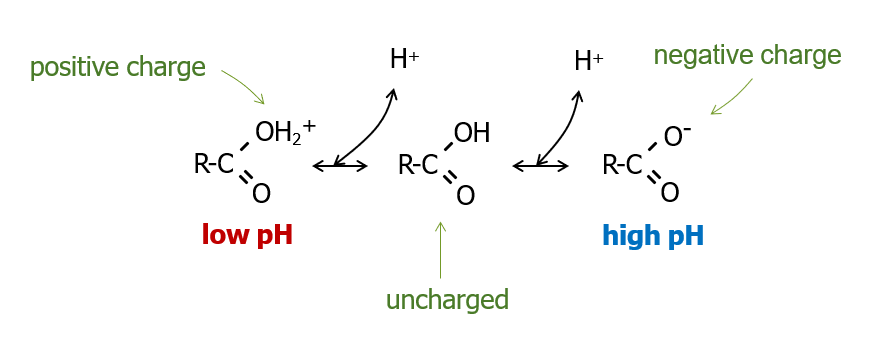
Here are some important points about the 3 non-phyllosilicate colloids in this chapter:
- Poorly crystalline silicates:
- Sphere- and tube-shaped minerals containing SiO4
- Form from volcanic ash
- Abundant in Andosols
- Very high water retention, moderate nutrient retention
- Oxides and hydroxides:
- Highly weathered minerals containing O2-, OH–, and OOH3-, bonded to transition and post-transition metals (mainly Fe and Al)
- Form from very extensive weathering of other minerals
- Lowest surface charge density of all of the colloids, resulting in the lowest nutrient retention of all of the colloids
- Abundant in highly weathered soils, found in tropical and subtropical regions
- Organic colloids:
- Particles of biological origin <1 μm in size that contain C-H and/or C-C bonds, also called “colloidal humus”
- Form from extensive decomposition of dead organisms, primarily plants
- Highest pH-dependent charge and nutrient retention of all of the colloids
- These 3 colloids all have pH-dependent charge, and no permanent charge

